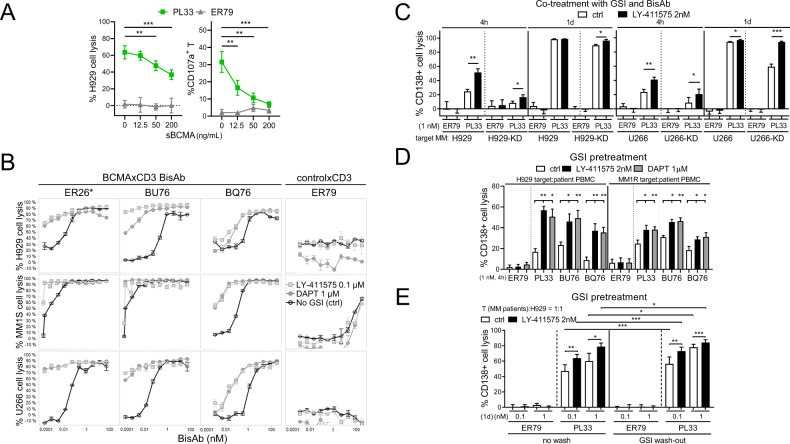
Fig. 2. BCMAxCD3 bispecific antibodies (BisAbs)-induced MM cytolysis was significantly enhanced by GSI, in the presence of MM patient effector cells.
A Serial dilutions of recombinant BCMA protein (sBCMA, 12.5–200 ng/mL) were added for 1 day in the co-cultures of patient T cells (n = 5) with H929 target MM cells (E:T = 1:1) in the presence of PL33 (BCMAxCD3, green square) vs. ER79 (non-targeting controlxCD3, gray triangle) BisAbs. Quantitative FC-based redirected T-cell cytotoxicity (RTCC) assays were used to determine % lysis of CD138+ target MM cells. Shown are summary data of % lysis of H929 cells (left) and CD107a degranulation on patient T cells (right). B Quantitative bioluminescence (BLI)-based RTCC assays were used to determine % lysis of 3 indicated MM target cells by T cells (n = 3) (E:T = 3:1) in the presence of 3 other BCMAxCD3 (ER26, BU76 or BQ76) clones vs. ER79, with or without 0.1 μM LY-411575 (gray square) or 1 μM DAPT (gray circle). No GSI control media (ctrl, black) was included. *ER26 has the same anti-BCMA Fab as PL33 used in all other figures. C–E Quantitative FC-based RTCC assays were used to determine % lysis of CD138+ target MM cells. C PL33 or ER79 (1 nM) were added for 4 h or 1 day in the co-culture of patient T cells (n = 5) with indicated paired MM target cells (control vector-transfected and BCMA-KD) (E:T = 3:1), in the presence of 2 nM LY-411575 or ctrl media. D H929 and MM1R target cells were pretreated with LY-411575 (2 nM) or DAPT (1 μM) followed by 4 h-co-incubation with MM patient PBMCs (n = 8) (E:T = 10:1) in the presence of indicated BisAbs (1 nM). (E) H929 target cells were pretreated with LY-411575 (2 nM) for 1 day. LY-411575 was then washed out (wash-out) or not (no wash) prior to 1 day-co-culture with MM patient T cells (n = 5) (E:T = 1:1) in the presence of PL33 vs. ER79. Multiple independent experiments using effector cells from MM patients (A n = 5; C n = 5; D n = 8; E n = 5) or normal donors (B, n = 3) were done with each treatment condition in triplicate. Data are presented as means ± SDs (error bars). *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001; ns, not significant.