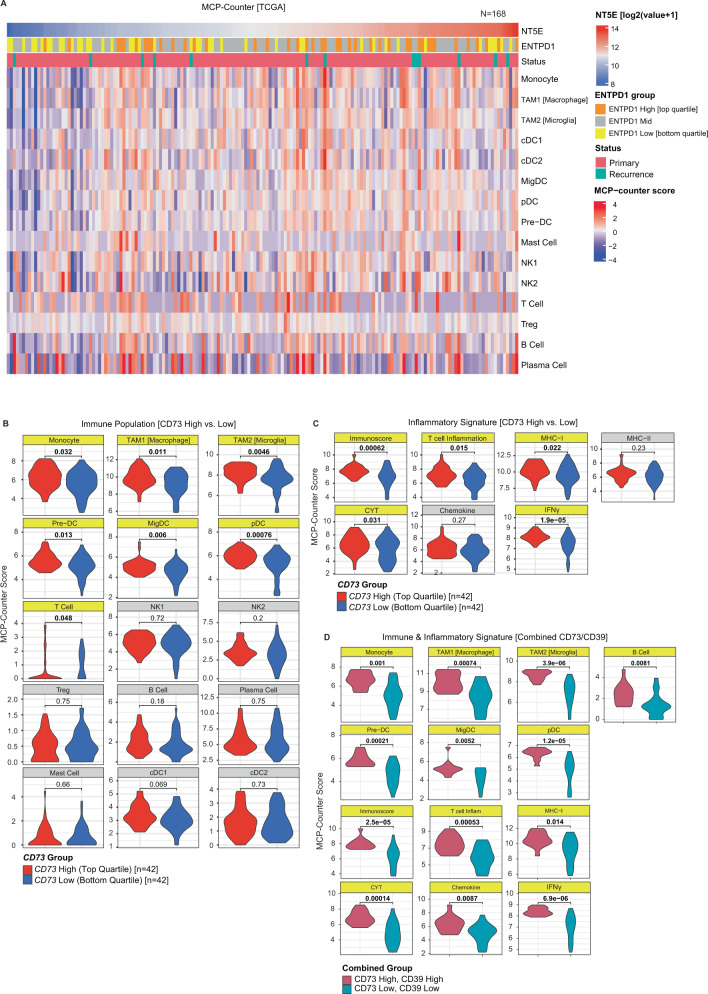
Fig. 3. CD73 and CD39 are associated with inflammatory signatures in glioblastoma.
A To better understand the inflammatory landscape of glioblastoma, bulk mRNA-sequencing data from 168 adult glioblastomas (TCGA) were deconvolved by Microenvironment Cell Population-Counter (MCP-Counter) to estimate the relative abundance of tumor and immune populations and correlate with purine regulatory enzymes and inflammatory signaling signatures. Analysis of all glioblastoma cases showed a positive correlation between CD73 and CD39 expression, and trend of greater numbers of most immune populations in CD73-high samples. B Comparison of cases with high CD73 expression (top-quartile, n = 42), and those with low expression (bottom-quartile, n = 42), showed that high levels were significantly (p < 0.05, Wilcoxon test, unpaired, two-sided) associated with a greater relative density of myeloid (naïve monocyte, TAM1 (macrophage), TAM2 (microglial)), dendritic (plasmacytoid dendritic cells (pDC) and pre-dendritic cells (pre-DC)), and T-cell populations, with no significant difference in NK, B cell, T regulatory cells, plasma cells, classical dendritic cell (cDC1/2), or mast cell populations. C Bulk analysis of gene signatures associated with inflammatory signaling showed that tumors with high CD73 expression exhibited significantly higher inflammation, including elevation of signatures associated with interferon-γ, MHC-I, Immunoscore, cytolytic (CYT), and T cell inflammatory pathways (p < 0.05, Wilcoxon test, unpaired, two-sided). D We noted that cases with coordinate expression of CD73 and CD39 showed particularly pronounced changes in immune populations and signaling. Direct comparison of CD73hiCD39hi tumors with CD73loCD39lo tumors showed an even more strongly significant relative enrichment of myeloid (monocyte, TAM1, TAM2), dendritic (pre-DC, migDC, pDC), and B cell populations in CD73hiCD39hi tumors) (p < 0.05, Wilcoxon test, unpaired, two-sided), as well as greater elevation of inflammatory signaling (T cell, MHC-I, IFN-γ, and chemokine) (p < 0.05, Wilcoxon test, unpaired, two-sided).