Fig. 7.
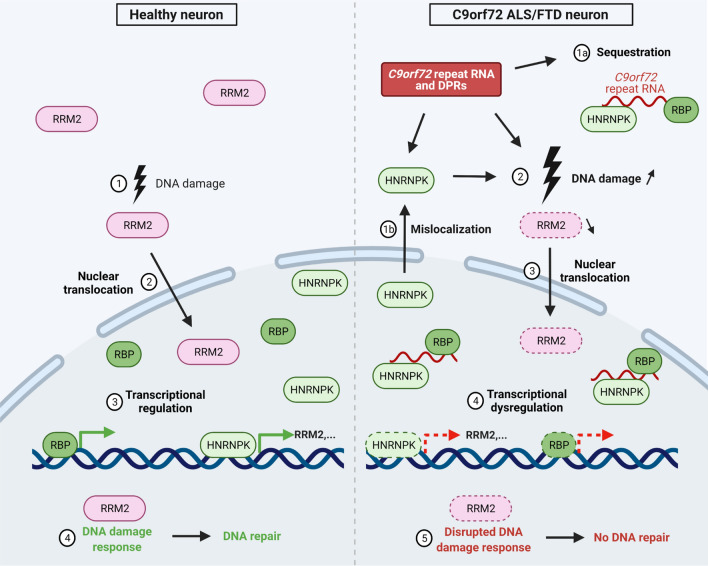
Schematic model linking mechanistic insights of HNRNPK and RRM2 in C9orf72 ALS. In the left panel, event of naturally occurring DNA damage (1) in a healthy neuron with consequential RRM2 activation and nuclear translocation (2). HNRNPK is a transcriptional regulator of RRM2 (3), essential for DNA repair in the DNA damage response (4). In the right panel, a neuron affected in C9 ALS/FTD is characterized by DPRs and RNA foci, consisting of C9orf72 repeat RNA and sequestered RNA-binding proteins, including HNRNPK (1a). Next to sequestration, HNRNPK is mislocalized to the cytoplasm (1b), resulting in a loss-of-function of HNRNPK, and directly or indirectly increasing DNA damage, which activates RRM2 (3). Loss-of-function achieves transcriptional dysregulation of downstream effectors of HNRNPK, including RRM2 (4). Activated, though depleted RRM2 results in a disrupted DNA damage response, impeding DNA repair (5). Scheme created with BioRender.com