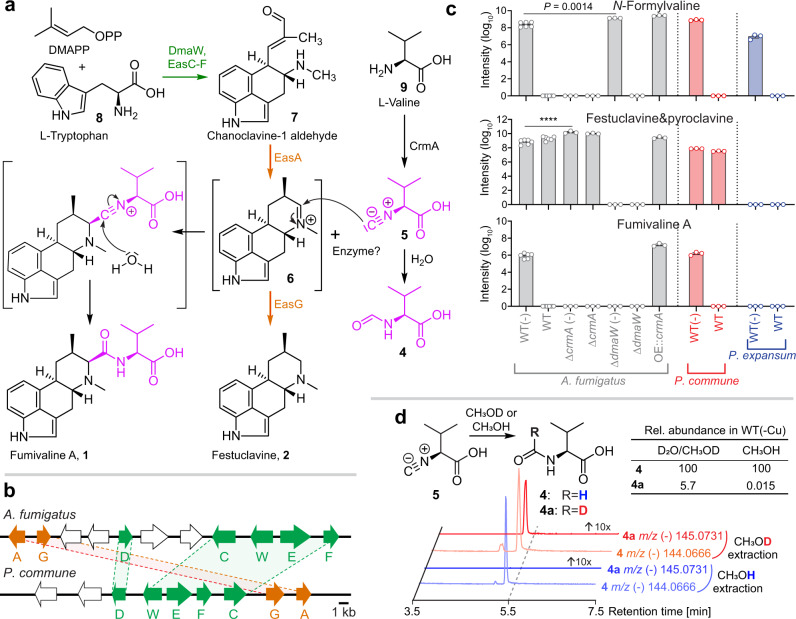
Fig. 2. Comparison of fumivaline BGCs and ergot alkaloids production.
a Ergot alkaloid biosynthesis pathways in A. fumigatus and P. commune. l-Tryptophan (8) and dimethylallyl pyrophosphate are converted into chanoclavine-1 aldehyde (7) by DmaW and EasC-F, which is then converted into festuclavine (2) by EasA and EasG in A. fumigatus and P. commune. Valine (9) is converted into valine isocyanide (5) by CrmA, which then reacts with the imine intermediate of festuclavine (6), resulting in formation of the amide bond in fumivaline A following hydration. In addition, hydration of valine isocyanide (5) produces N-formylvaline (4). b Comparison of crm BGCs of A. fumigatus and P. commune. Homologous genes are marked using the same colors as in a. A and C-G represent easA and easC-G respectively. W represents dmaW. c Relative abundances of N-formylvaline (4), fumivaline A (1), and festuclavine (2) and pyroclavine in A. fumigatus, P. commune, and P. expansum (gray, red, and blue, respectively) grown without copper (-) or with copper. Bars represent mean ± s.e.m. with six independent biological replicates for A. fumigatus wildtype and three for the other strains. p values were calculated by unpaired, two-sided t-test with Welch’s correction, ****P < 0.0001. d Relative abundances of N-formylvaline (4) and [7-2H]-N-formylvaline (4a) in extracts of wildtype A. fumigatus (grown without copper) extracted with deuterated or non-deuterated solvents. Source data are provided as a Source Data file.