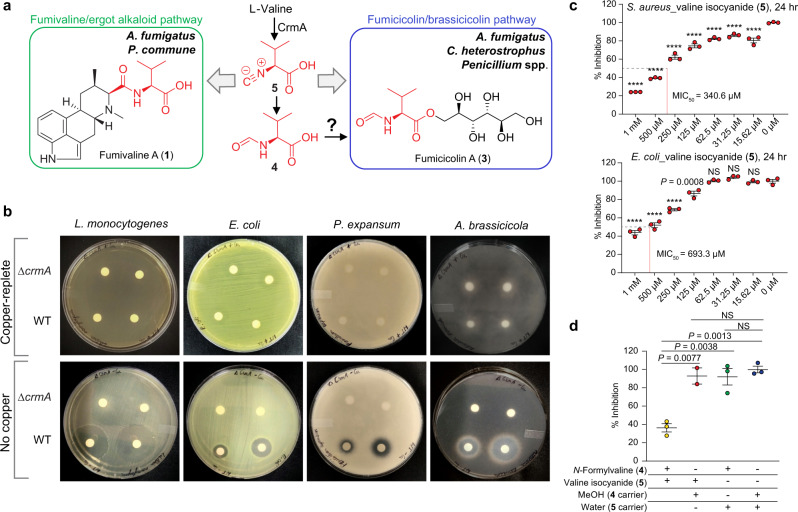
Fig. 4. Putative biosynthesis of CrmA-derived metabolites in diverse fungi and antimicrobial activities.
a Fumivaline biosynthesis was observed in A. fumigatus and P. commune, which has the ergot alkaloid BGC and a homolog for crmA, whereas fumicicolin biosynthesis was observed in A. fumigatus, Penicillium spp. and C. heterostrophus, all of which harbor crmA homologs. b Growth of Listeria monocytogenes, Escherichia coli, Pencillium expansum, and Alternaria brassicicola is inhibited when challenged with extracts from WT but not ΔcrmA A. fumigatus grown without copper supplementation. Extracts from copper supplemented cultures do not inhibit microbial growth. c Valine isocyanide (5) significantly inhibits the growth of Staphylococcus aureus at all concentrations tested and inhibits the growth of E. coli at 125 μM and higher. MIC50, minimum inhibitory concentration to inhibit 50% growth. d Valine isocyanide (5) and N-formylvaline (4) show synergistic antifungal activity against Candida auris at 36 h. In c and d, bars represent mean ± s.e.m. with three independent biological replicates. One-way ANOVA with Dunnett’s multiple comparisons test was performed to assess if the differences in survival at the range of concentrations were statistically significant (at p < 0.05) from survival with solvent only (0 µM), ****p < 0.0001, NS, not significant. Source data are provided as a Source Data file.