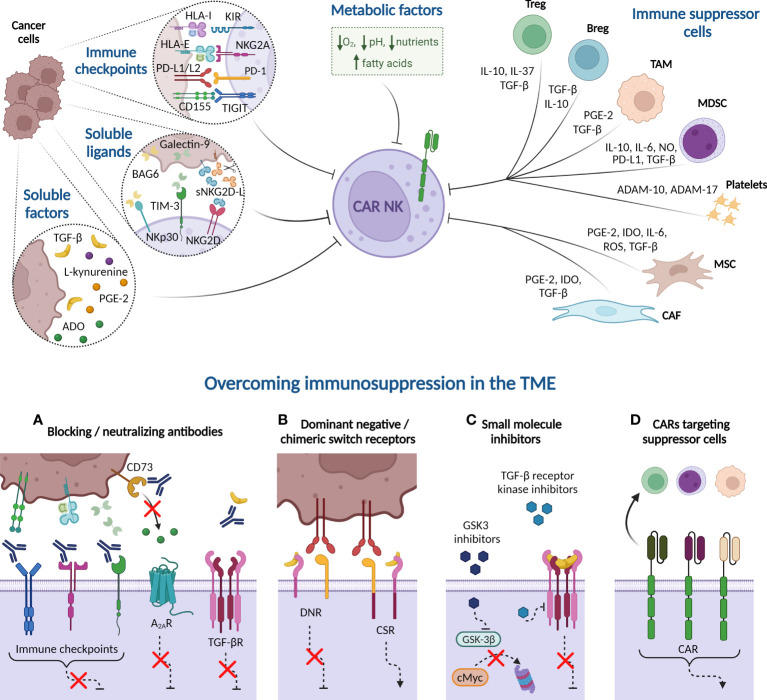
Figure 3.
Immune suppressive tumor microenvironment (TME) factors potentially involved in CAR-NK cell dysfunction. Cancer cells express immune checkpoint ligands in their plasma membrane that mediate an inhibitory interaction with NK cells. Besides, tumor cells may suppress NK cell function by releasing soluble ligands to the milieu, such as BAG-6, galectin-9, and soluble NKG2D-L (sNKG2D-L), as well as other soluble factors, including cytokines, such as transforming growth factor-β (TGF-β), enzymes and metabolites. Many of these soluble factors are also produced by immune cells present in the TME, such as Tregs, Bregs, tumor-associated macrophages (TAM), and myeloid-derived suppressor cells (MDSC). Platelets, in turn, secrete the metalloproteinases ADAM-10 and ADAM-17 that prompt NKG2D-L shedding. Other non-immune cells, such as derived-mesenchymal stromal cells (MSC) and cancer-associated fibroblasts (CAF), also produce indoleamine 2, 3 dioxygenase (IDO) or reactive oxygen species (ROS) that reduce NK cell activity. Additionally, hypoxia, high concentrations of fatty acids, nutrient deprivation, and acidity, among other metabolic factors, contribute to generate a complex immunosuppressive TME that hampers the NK cell effectiveness against hematologic malignancies. Several strategies can overcome the immunosuppression mechanism from TME. (A) Blocking antibodies targeting immune checkpoints prevent the inhibition of NK cell cytotoxicity. Other receptors, such as adenosine A2A receptor (A2AR) also disable NK cell function when binds to extracellular adenosine (ADO). Blockade of CD73 ectoenzyme, which synthetizes ADO, reduces the levels of this metabolite in the TME, therefore increasing NK cell killing activity. Furthermore, anti-TGF-β neutralizing antibodies impede the NK cell suppressive effect unleashed by the interaction of this cytokine with its receptor (TGF-βR). (B) Dominant-negative receptor (DNR) expression hinders the inhibitory signaling triggered by PD-1 and TGF-βR in the presence of PD-L1/L2 or TGF-β, respectively. Chimeric switch receptors (CSR) constitutes another approach based on replacing these negative signals by activating ones, through intracellular domains exchange, reverting the outcomes in NK cell activity. (C) Small molecule inhibitors directed against GSK-3β impact on NK cell metabolism and improve their cytotoxic potency. Other inhibitors are engineered to inhibit the kinase activity of TGF-βR. (D) CAR constructs are designed against molecules expressed in immune suppressor cells to eliminate them from TME. HLA-I, HLA class I histocompatibility antigen; KIR, Killer-cell immunoglobulin-like receptor; HLA-E, HLA class I histocompatibility antigen, alpha chain E; PD-L1, Programmed Death ligand-1; PD-1, Programmed Death 1; TIGIT, T cell immunoglobulin and ITIM domain; BCL2-associated Athanogene 6 (BAG-6); sNKG2D-L, soluble natural killer group 2D ligands; TIM-3, T cell immunoglobulin and mucin-domain containing-3; PGE-2, prostaglandin E2; NO, nitric oxide; A Disintegrin And Metalloproteinase (ADAM). Created with BioRender.com.