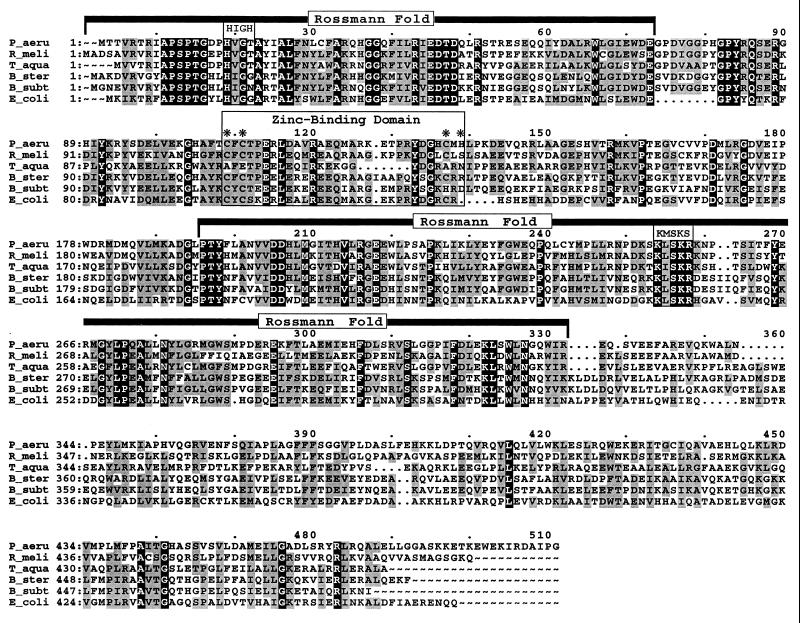
FIG. 2.
Multiple-sequence alignment of GltX. The predicted protein product from P. aeruginosa gltX was compared to five other known glutamyl-tRNA synthetases by using the Pileup program from the Genetics Computer Group (Madison, Wis.) package (Wisconsin Package, version, 9.1). Comparisons were made with the blosum 62 matrix, a gap weight of 12, and a gap length weight of 4. The GltX sequences used were as follows: P_aeru, P. aeruginosa PA103 (this study); R_meli, R. meliloti (GenBank accession no. P15189); T_aqua, Thermus aquaticus (GenBank accession no. P27000); B_ster, Bacillus stearothermophilus (GenBank accession no. P43818); B_subt, B. subtilis (GenBank accession no. P22249); E_coli, E. coli (GenBank accession no. P04805). Residues which were identical in all six sequences are printed in white on black, while those conserved in at least four of the six sequences are shaded. The positions of the Rossmann fold, including the HIGH and KMSKS motifs, and the zinc-binding domain are indicated. The locations of the four residues implicated in coordinating zinc in the E. coli glutamyl-tRNA synthetase are marked with asterisks.