Table 1.
Physicochemical data of 2-(1-isonicotinoyl-3-aryl-4,5-dihydro-1H-pyrazol-4-yl)-3-phenyl thiazolidin-4-one derivatives P (1–21)
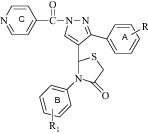
| |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Comp | R | R1 | Mol. Wt | Rf* | Rf** | Conventional synthesis | Microwave-assisted synthesis | Melting point (°C) | |||
| Reaction Time (hrs) | % yield | Microwave power (W) | Reaction time (min) | % yield | |||||||
| P-1 | 4-NO2 | H | 473 | 0.60 | 0.58 | 1.0 | 68.2 | 300 | 2 | 89.2 | 244–246 |
| P-2 | 4-NO2 | 4-NO2 | 520 | 0.37 | 0.50 | 1.0 | 67.3 | 300 | 2 | 87.4 | 266–268 |
| P-3 | 4-NO2 | 2-NO2 | 520 | 0.20 | 0.32 | 1.0 | 60.5 | 300 | 2 | 83.1 | 282–284 |
| P-4 | 4-NO2 | 4-Cl | 507 | 0.23 | 0.24 | 1.0 | 56.9 | 300 | 2 | 80.0 | 286–288 |
| P-5 | 4-NO2 | 3-Cl | 507 | 0.69 | 0.64 | 1.0 | 59.2 | 300 | 2 | 75.8 | 274–276 |
| P-6 | 4-NO2 | 3-OCH3 | 503 | 0.71 | 0.67 | 1.0 | 61.2 | 300 | 2 | 72.6 | 262–264 |
| P-7 | 4-NO2 | 2-OCH3 | 503 | 0.66 | 0.62 | 1.0 | 67.4 | 300 | 2 | 93.4 | 250–252 |
| P-8 | 4-OCH3 | H | 456 | 0.55 | 0.63 | 1.0 | 73.7 | 300 | 2 | 82.5 | 300–302 |
| P-9 | 4-OCH3 | 4-NO2 | 503 | 0.53 | 0.71 | 1.0 | 68.9 | 300 | 2 | 76.3 | 308–310 |
| P-10 | 4-OCH3 | 2-NO2 | 503 | 0.47 | 0.65 | 1.0 | 56.4 | 300 | 2 | 64.7 | 322–324 |
| P-11 | 4-OCH3 | 4-Cl | 490 | 0.35 | 0.70 | 1.0 | 59.2 | 300 | 2 | 68.8 | 318–320 |
| P-12 | 4-OCH3 | 3-Cl | 490 | 0.48 | 0.58 | 1.0 | 61.6 | 300 | 2 | 72.4 | 338–340 |
| P-13 | 4-OCH3 | 3-OCH3 | 486 | 0.63 | 0.64 | 1.0 | 66.8 | 300 | 2 | 76.6 | 320–322 |
| P-14 | 4-OCH3 | 2-OCH3 | 486 | 0.32 | 0.59 | 1.0 | 73.9 | 300 | 2 | 84.3 | 336–338 |
| P-15 | 4-Br | H | 504 | 0.70 | 0.65 | 1.0 | 66.8 | 300 | 2 | 84.7 | 256–258 |
| P-16 | 4-Br | 4-NO2 | 551 | 0.40 | 0.36 | 1.0 | 58.2 | 300 | 2 | 68.9 | 268–270 |
| P-17 | 4-Br | 2-NO2 | 551 | 0.21 | 0.35 | 1.0 | 61.2 | 300 | 2 | 72.0 | 252–254 |
| P-18 | 4-Br | 4-Cl | 538 | 0.74 | 0.79 | 1.0 | 59.7 | 300 | 2 | 79.8 | 244–246 |
| P-19 | 4-Br | 3-Cl | 538 | 0.70 | 0.74 | 1.0 | 64.2 | 300 | 2 | 72.3 | 248–250 |
| P-20 | 4-Br | 3-OCH3 | 535 | 0.60 | 0.65 | 1.0 | 61.3 | 300 | 2 | 83.2 | 252–254 |
| P-21 | 4-Br | 2-OCH3 | 535 | 0.28 | 0.32 | 1.0 | 58.4 | 300 | 2 | 69.1 | 260–262 |
TLC Mobile phase: *n-hexane: ethylacetate (4:6), **Ethylacetate: n-hexane: methanol (3:6:1)
