Fig. 2.
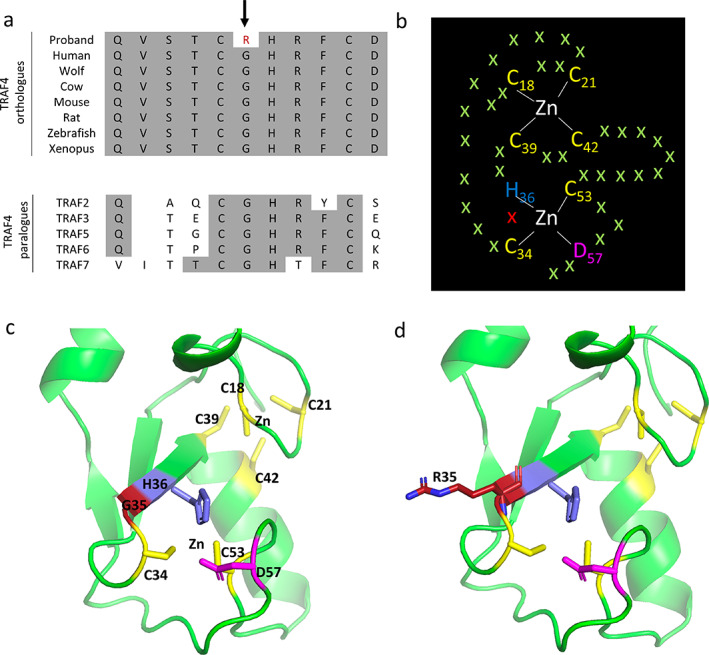
Evolutionary conservation of TRAF4 Gly35, and structural analysis of the Gly35Arg variant. (A) Multiple protein sequence alignment of TRAF4 revealed evolutionary conservation of Gly35 (G35) residues (indicated with an arrow) in orthologues and human paralogues. Conserved residues are shaded gray. In addition, the CGHRFC motif within the RING domain of the TRAF family of proteins is largely conserved in TRAF4 paralogues. (B) The RING domain of TRAF4 is predicted to contain two zinc (Zn) binding pockets involving six cysteine (C) residues (18, 21, 34, 39, 42, 53), one histidine (H) residue (36), and one aspartic acid (D) residue (57). G35 is shown as a red cross. (C) AlphaFold prediction of TRAF4 (AF‐Q9BUZ4‐F1) using PyMOL analysis showing the RING domain and the orientation of residues around the two zinc binding pockets. (D) PyMOL analysis mutating the neutrally charged small glycine residue at codon 35 to a positively charged larger arginine (R) residue, illustrating its close proximity to the zinc binding pocket, which may affect the ability of the TRAF4 RING domain to bind zinc and function as an E3 ligase.
