Table B.1.
Summary of Safety Evaluation by JECFA of the substances in FGE.19 subgroup 3.3, applying the Procedure (based on intakes calculated by the MSDI approach) (JECFA, 2006, 2015)
|
FL‐no JECFA‐no CAS no |
EU Register name | Structural formula | MSDI (a) (μg/capita per day) |
Class (b) Evaluation procedure path (c) (JECFA) |
Outcome on the named compound (JECFA, 2006) | Outcome on the named compound (JECFA, 2015) | EFSA conclusion on the named compound (genotoxicity) |
|---|---|---|---|---|---|---|---|
|
05.062 1474 4411‐89‐6 |
2‐Phenylcrotonaldehyde |
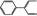
|
1.7 |
Class I A3: Intake below threshold |
(d) | (e) | Concern for aneugenicity cannot be ruled out at the reported use levels. |
|
05.099 1472 21834–92‐4 |
5‐Methyl‐2‐phenylhex‐2‐enal |
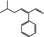
|
15 |
Class II A3: Intake below threshold |
(d) | (e) | Concern for aneugenicity cannot be ruled out. Additional genotoxicity data required. |
|
05.100 1473 26643–91‐4 |
4‐Methyl‐2‐phenylpent‐2‐enal |
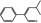
|
0.34 |
Class II A3: Intake below threshold |
(d) | (e) | Concern for aneugenicity cannot be ruled out. Additional genotoxicity data required. |
|
05.175 – 3491‐63‐2 |
2‐Phenylpent‐2‐enal |
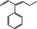
|
0.011 | No evaluation | Not evaluated by JECFA | Not evaluated by JECFA | Concern for aneugenicity cannot be ruled out. Additional genotoxicity data required. |
|
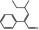
05.222 2069 26643–92‐5 |
2‐Phenyl‐4‐methyl‐2‐hexenal |
|
3.0 | No evaluation | Not evaluated by JECFA | (e) | Concern for aneugenicity cannot be ruled out. Additional genotoxicity data required. |
EU MSDI: Amount added to food as flavour in (kg/year) × 10E9/(0.1 × population in Europe (= 375 × 10E6) × 0.6 × 365) = μg/capita per day, calculated by EFSA based on the most recent data submitted.
Thresholds of toxicological concern: Class I = 1,800 μg/person per day, Class II = 540 μg/person per day, Class III = 90 μg/person per day.
Procedure path A substances can be predicted to be metabolised to innocuous products. Procedure path B substances cannot.
No safety concern based on intake calculated by the MSDI approach of the named compound.
The Procedure cannot be applied until concerns regarding genotoxicity of 2‐phenyl‐2‐butenal (JECFA No. 1474) are resolved and the evaluation should be reconsidered at a future meeting.
