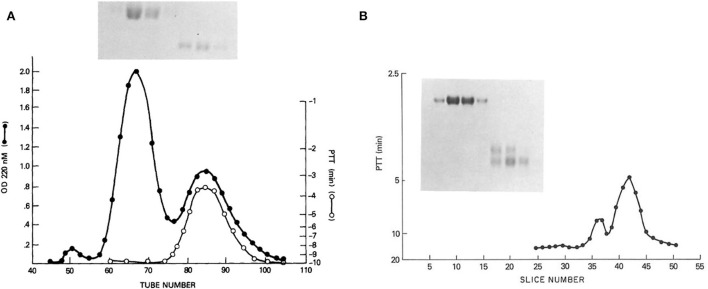
Figure 1.
(A) Sephadex G-200 gel filtration of reduced kinin-free HMW kininogen. The absorbance at 280 nm is shown (black line) in addition to the ability of fractions to correct the partial thromboplastin time of HK kininogen-deficient plasma (white line). Above (inset) is shown the SDS-PAGE pattern obtained after electrophoresis of 100 μl of tubes 60, 65, 70, and 75 (heavy chain), and electrophoresis of 100 μl of a 10-fold concentration of tubes 80, 85, and 90 (light chain). (B) Urea disc gel electrophoresis of samples taken from the Sephadex G-200 gel filtration of reduced kinin-free HWM kininogen [in this figure (A)]. A 100 μl of tubes 60, 65, 70, and 75 followed by 100 μl of a 20-fold concentration of tubes 80, 85, and 90 were applied. A replicate gel was sliced, each slice was eluted with 0.2 ml phosphate-buffered saline, and the eluates were assayed for their ability to correct the partial thromboplastin time of HMW kininogen-deficient plasma. The peaks of coagulant activity seen at slices 36–38 and 40–44 correspond to the two fainter light chain bands seen on the right side of the gel.