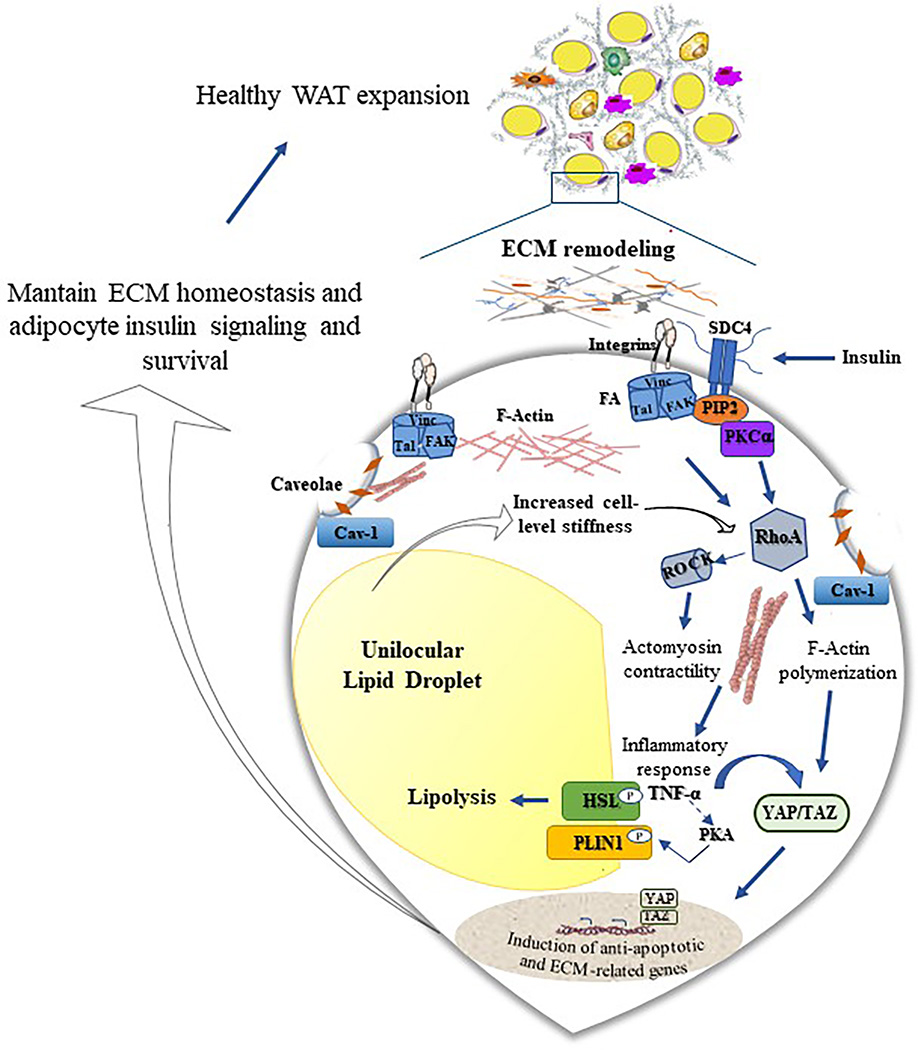
Figure 1. Schematic representation of the mechanosensing machinery in the adipocyte and its impact on white adipose tissue (WAT) expansion.
Mechanical properties of the ECM are sensed by the adipocyte via a range of membrane-bound mechanosensors, including integrins, syndecan 4 (SDC4), focal adhesion (FA) complex proteins, and Caveolin-1 (Cav-1), the principal component of caveolae in adipocytes. These mechanosensors dynamically regulate the transfer of extracellular/intracellular mechanical signals inside/outside the cell. ECM-mediated activation of the FA protein vinculin (Vinc), talin (Tal), and focal adhesion kinase (FAK) produces downstream signaling events regulating the activity of Rho family member A (RhoA). RhoA activation, which is also mediated by increased cell-level stiffness and SDC4-induced phosphorylation of extracellular signal-regulated kinase, leads to activation of the Ras homologous (Rho)/Rho-associated kinase (ROCK) signaling pathway and a consequent increase in tension of the actomyosin cytoskeleton. This promotes the nuclear translocation of the transcriptional factors Yes-associated protein (YAP) and transcriptional co-activator with PDZ-binding motif (TAZ) resulting in transcriptional control of genes that sustain ECM homeostasis and adipocyte survival. The increased cellular tension also induces the expression of tumor necrosis factor-α (TNFα), which in turn, stimulates lipolysis through activation of cAMP-dependent protein kinase A (PKA) and its phosphorylation of perilipin-1 (PLIN1) and hormone-sensitive lipase (HSL). The interaction between phosphorylated PLIN1 and HSL allows HSL to translocate from the cytosol to the lipid droplet where it promotes lipolysis. Activation of lipolysis may, in turn, counterbalance the adipocyte hypertrophy-induced cellular tension.