Abstract
Purpose:
To evaluate the changes in T-cell balance in peripheral blood following percutaneous tumor ablation.
Material and methods:
Patients underwent thermal ablation including radiofrequency (n = 9) and microwave ablation (n = 5), or cryoablation (n = 5). Target tumors were located in the lung (n = 7), soft tissue (n = 5), liver (n = 4), and bone (n = 3). Patient peripheral blood samples were collected before and within 14 days after ablation. Peripheral blood populations of cytotoxic T-cells (CTL), type-1 (Th1) and type-2 helper T-cells (Th2), and regulatory T-cells (Treg) were measured using flow cytometry. Changes in CTL/Treg and Th1/Th2 ratios before and after ablation therapy were compared using paired t-tests.
Results:
Peripheral blood CTL population (27.5 ± 2.1% to 30.2 ± 2.5%, p < .03) and CTL/Treg ratios (18.8 ± 3.7% to 21.6 ± 3.6%, p < .05) increased significantly after ablation. Although a significant increase in CTL/Treg ratios was found after heat-based ablation (18.0 ± 4.4% to 21.6 ± 4.7%, p < .02), it remained unchanged after cryoablation (21.0 ± 7.0% to 21.5 ± 4.3%, p = .92). Th1/Th2 ratio (13.7 ± 3.0% to 17.2 ± 3.5%, p = .12) remained unchanged after ablation.
Conclusion:
Ablation therapy alters the T-cell balance by increasing the systemic CTL/Treg, ratio. Heat-based ablation might be a more effective approach than cryoablation to enhance systemic anti-tumor immunity.
Keywords: Radiofrequency ablation, microwave ablation, cryoablation, T-cell, lymphocyte
Introduction
Percutaneous ablation therapy has been performed increasingly for the treatment of solid tumors in various organs (1). The clinical efficacy of ablation therapy primarily emphasizes its direct tumoricidal effect. More recently, the indirect effects of ablation therapy have also received attention (2). Ablation therapy destroys the tumor in situ and creates an antigen source for the generation of anti-tumor immunity (3–5). Ablation therapy also induces cytokine production, which can alter the local and systemic immune environment (6–8).
Previous reports of the literature showed that concentrations of cytokines such as interleukin (IL)-6 and IL-10 increase significantly after thermal ablation (6–8). Given that both IL-6 and IL-10 affect T-cell differentiation (9,10), we hypothesized that ablation therapy changes the T-cell balance. Understanding the T-cell balance alteration is important because there is accumulating evidence that some T-cell subtypes promote the death of cancer cells (cytotoxic T-lymphocytes [CTL] and type-1 helper T-cells [Th1]), whereas other T-cell classes promote the survival of cancer cells (type-2 helper T-cells [Th2] and regulatory T-cells [Treg]) (11,12). Changes in T-cell balance are also known to affect disease progression and patient prognosis (13–18).
Nevertheless, details of the influence of ablation therapy on T-cell balance have not been evaluated. Therefore, this study evaluated changes in T-cell balances in patients’ peripheral blood following ablation therapy.
Material and methods
Patients
Our institutional review board approved this prospective study. Written informed consent was obtained from all patients. Patients who were scheduled to receive percutaneous ablation therapy for the treatment of primary or secondary malignancy were included in this study. Exclusion criteria included prior ablation or embolization procedure within 30 days, combination with other cancer treatments such as chemotherapy and immunotherapy, and steroids medication within 30 days before study enrollment. Patients who did not require clinical blood testing before the ablation procedures were also excluded.
Between January 2014 and December 2014, five interventional radiologists and one research assistant recruited the study participants. Consequently, 41 patients were enrolled in this study. Later, one patient withdrew from the study. Furthermore, 19 patients were excluded because scheduled laboratory testing was not performed after the procedure. The blood sample amounts of two other patients were insufficient for analysis, leaving 19 patients, who formed the cohort of this study. They were nine women (47.4%) and ten men (52.6%), with a mean age of 59 ± 3.5 (range 21–81) years. The most common primary diagnoses were colorectal cancer (42.1%, 8/19) and soft tissue tumor (42.1%, 8/19) including leiomyosarcoma (n = 4), desmoid (n = 2), gastrointestinal stromal tumor (n = 1), and solitary fibrous tumor (n = 1). Fourteen patients underwent heat-based ablation including radiofrequency (n = 9) and microwave (n = 5) ablation, or cryoablation (n = 5).Table 1 presents patient background information and tumor characteristics.
Table 1.
Patient backgrounds and tumor characteristics.
| Parameter | Value |
|---|---|
| Patient no. | 19 |
| Age (years) | 59 ± 3 |
| ≦60 | 8 (42.1) |
| >60 | 11 (57.9) |
| Gender | |
| Male | 10 (52.6) |
| Female | 9 (47.4) |
| Primary diagnosis | |
| Colorectal | 8 (42.1) |
| Soft tissue | 8 (42.1) |
| Lung | 2 (10.5) |
| Gynecologic | 1 (5.3) |
| Target tumor | |
| Primary | 3 (15.8) |
| Metastasis | 16 (84.2) |
| Ablation site | |
| Lung | 7 (36.8) |
| Soft tissue | 5 (26.3) |
| Liver | 4 (21.1) |
| Bone | 3 (15.8) |
| Target tumor number | |
| Single | 16 (84.2) |
| 2 or 3 | 3 (15.8) |
| Maximum diameter of target tumor (cm) | 2.0 ± 0.3 |
| ≦2 | 13 (68.4) |
| >2 | 6 (31.6) |
| Ablation modality | |
| Heat-based ablation | 14 (73.7) |
| Cryoablation | 5 (26.3) |
| Ablative zone area (cm2) | 11.9 ± 2.7 |
| ≦10 | 11 (57.9) |
| >10 | 8 (42.1) |
Continuous data are presented as mean and standard error (SE). Numbers in parentheses are percentages.
Percutaneous tumor ablation
Percutaneous tumor ablation was performed by five interventional radiologists. All thermal ablation procedures were performed under general anesthesia. A single dose of prophylactic antibiotics (Ancef; GlaxoSmithKline, Research Triangle Park, NC, USA) was administered intravenously before the procedure. Applicators for ablation were placed under computed tomography (CT) or magnetic resonance imaging (MRI) guidance. Heat-based ablations were performed using Cool-tip (Covidien, Dublin, Ireland) or Certus (NeuWave Medical Inc., Madison, WI, USA) systems. Cryoablation was performed using the Endocare system (HealthTronics Inc., Austin, TX, USA). After the ablation applicators were positioned appropriately, ablation was performed. The endpoint of an ablation was marked by the creation of an ablation zone with a margin of at least 5 mm circumferentially around the tumor in all cases (19). If necessary, the ablation applicator was repositioned. Then additional ablations were performed to achieve the desired endpoint.
Immediately after the ablation, a non-contrast enhanced CT (for lung) or a contrast-enhanced CT (for liver and other sites) was performed to confirm the ablative zone. The ablative zone area was defined as the maximum area of the zone of non-enhancement on contrast-enhanced CT after the procedure. In the case of a lung ablation, the ablative zone area served as the size of ground-glass or lung consolidation after the procedure (as measured on axial imaging). In the case of multiple sites of ablation, the ablative zone areas of respective ablation sites were summed.
Blood collection
Patient peripheral blood was collected within one month before ablation (mean ± standard deviation 4.5 ± 1.4 days; range 0–21 days) and within 14 days (5.7 ± 5.1 days; range 1–14 days) after ablation. Assessments of white blood counts (WBCs) were conducted at our clinical chemistry laboratories using fresh blood samples. For the analysis of T-cell subtypes, peripheral blood mononuclear cells (PBMCs) were isolated by density gradient centrifugation medium (Ficoll-Paque; GE Healthcare Bio-Sciences, Piscataway, NJ, USA) from heparinized blood. Then, PBMCs were frozen immediately with freezing medium (Cell Freezing Medium-DMSO 1 ×; Sigma Aldrich Corp., St. Louis, MO, USA) at −80 °C and were stored until analysis.
Analysis of T-cell populations
Three researchers conducted analyses of T-cell populations. After thawing of frozen samples, 2 × 106 PBMCs were prepared for each analysis. For the analyses of CTL and Treg populations, 1 × 106 PBMCs were used. The PBMCs were first incubated for surface staining with the following antibodies: cluster of differentiation (CD) 4 PerCP-Cy5.5 (clone SK3; BD Biosciences, San Jose, CA), CD8 fluorescein isothiocyanate (FITC) (clone HIT8a; BD Pharmingen, San José, CA, USA), and CD25 (clone 4E3; Miltenyi Biotec, Auburn, CA, USA). After washing the cells using phosphate-buffered saline with 2% fetal bovine serum, intracellular staining by forkhead box P3 (FoxP3) phycoerythrin (PE) (clone 236 A/E7; eBioscience, San Diego, CA) was performed according to instructions provided by the manufacturer (Anti-Human FOXP3 Staining Set PE; eBioscience).
The remaining 1 ×106 PBMCs were used for the analyses of Th1 and Th2. After the surface staining with antibodies for CD4 PreCP-Cy5.5, cells were stimulated with 10 ng/mL of phorbol myristate acetate (Sigma-Aldrich Corp., St. Louis, MO, USA) and 1 ng/ml of ionomycin (Sigma-Aldrich Corp., St. Louis, MO, USA) in the presence of 0.67 μl/mL of protein transport inhibitor containing monensin (BD Golgistop; BD Bioscience) for 4 h at 37 ° C in an atmosphere containing 5% CO2. Then, intracellular staining was performed for interferon-gamma (IFN-γ) FITC (Clone B27; BD Pharmingen) and interleukin-4 (IL-γ) allophycocyanin (APC) (Clone MP4–25D2; BD Pharmingen).
After surface and intracellular staining, populations of each T-cell subset were analyzed on a flow cytometer (FACSCalibur; Becton, Dickinson and Co., Franklin Lakes, NJ, USA) (Figure 1(a–c)). A total number of 20,000 lymphocytes were obtained from each sample, after the populations of lymphocytes were gated from PBMCs according to the forward scatter and side scatter characteristics. This process was followed by the gating of the CD4+ and CD8+ subsets (Figure 1(a)). Populations of CD25+ FoxP3+, IFN-γ+, and IL-4+ cells were further evaluated using CD4+ gate (Figure 1(b,c)). CTL, Treg, Th1, and Th2 were defined respectively as CD8+, CD4+ CD25+ FoxP3+, CD4+IFN-γ+, and CD4+IL-4+ lymphocytes. All flow cytometry analyses were conducted using software (BD CellQuest; Becton, Dickinson and Co.)**.
Figure 1.
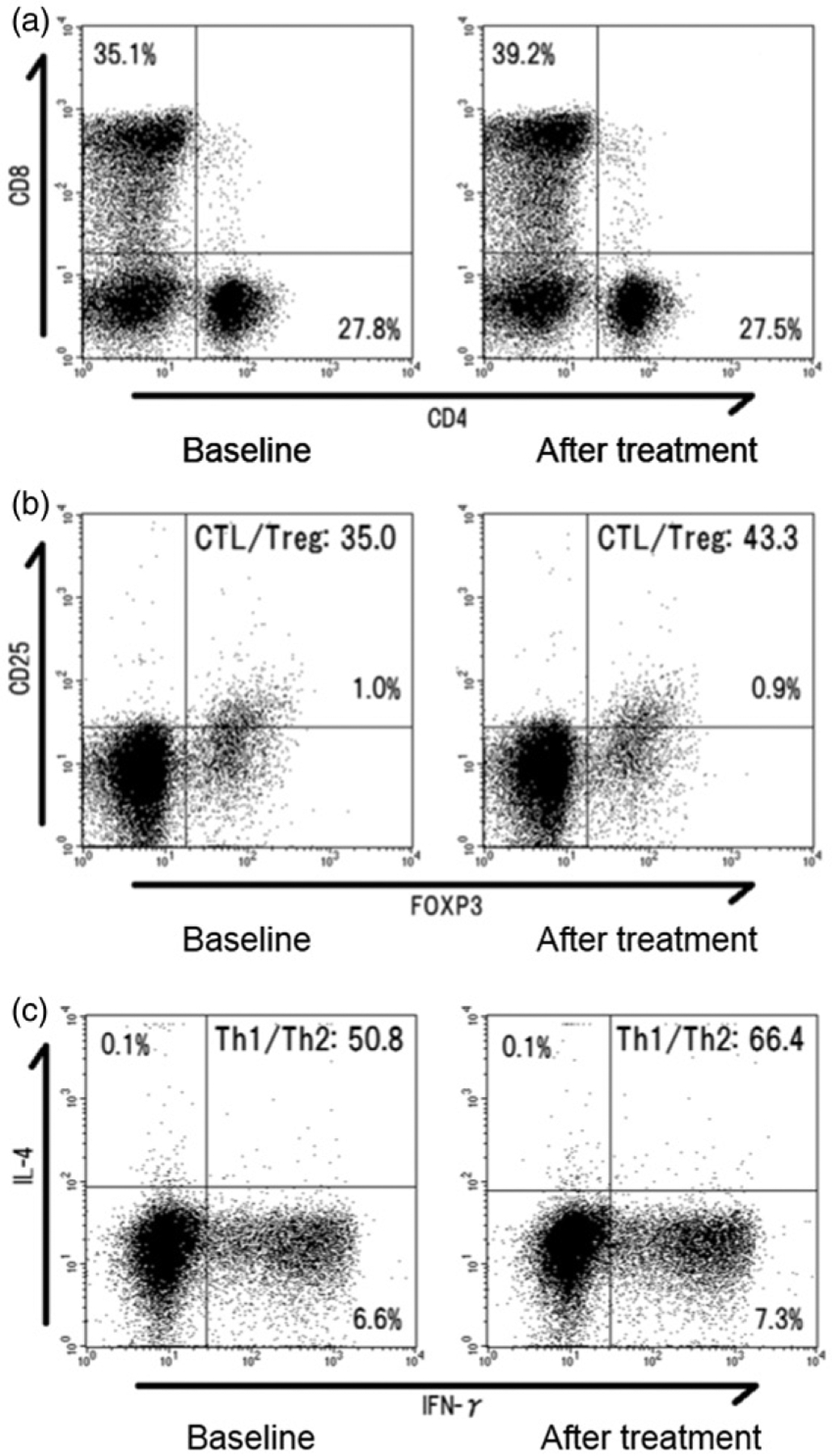
Representative flow cytometry results for a patient who received heat-based ablation for the treatment of lung metastasis from colorectal cancer. (a) Population of peripheral blood cytotoxic T-lymphocyte (CTL) changed from 35.1% to 39.2% at three days after ablation. (b) The CTL/regulatory T cell (Treg) ratio increased from 35.0% to 43.3% after ablation.(c) In this case, type-1 (Th1)/type-2 helper T-cells (Th2) ratio also increased 6.6% to 7.3% after ablation.
Measurements and statistical analysis
For this study, we evaluated the change in peripheral blood WBCs, neutrophils, and lymphocytes as well as the percentages of T-cell subtypes within the lymphocytes including CTL, Treg, and helper T-cells (Th1 and Th2) after ablation therapy. Furthermore, changes in T-cell balances including CTL/Treg and Th1/Th2 ratios were evaluated. Sub-analyses of changes in T-cell balances were also performed according to patient background, tumor characteristics, and ablation modality. Data are expressed as a mean and standard error (SE). Comparisons of samples between time points were made using a paired t-test. All p < .05 were inferred as statistically significant. All statistical analyses were performed using software (SAS, release 9.1; SAS Institute Inc., Cary, NC, USA).
Results
Changes in white blood cell, neutrophil, and lymphocyte counts
Changes in WBC, neutrophil, and lymphocyte counts are presented in Table 2. WBC counts (6.0 ± 0.5 to7.8 ± 0.8 × 103/μL, p < .02), neutrophil populations (65.7 ± 2.0% to 72.9 ± 2.8%, p < .01), and neutrophil counts (4.0 ± 0.4 to 5.9 ± 0.8 × 103/μL, p < .01) increased significantly after ablation therapy. However, lymphocyte populations (22.9 ± 2.0% to 17.5 ± 2.4%, p < .01) decreased significantly after ablation therapy. Lymphocyte counts (1.4 ± 0.2 to 1.2 ± 0.2 × 103/μL, p = .07) remained unchanged.
Table 2.
Changes in WBCs, neutrophils, and lymphocytes after thermal ablation.
| Baseline | After treatment | p value | |
|---|---|---|---|
| WBCs (×103/μL) | 6.0 ± 0.5 | 7.8 ± 0.8 | <.02 |
| Neutrophil (%) | 65.7 ± 2.0 | 72.9 ± 2.8 | <.01 |
| Neutrophil (×103/μL) | 4.0 ± 0.4 | 5.9 ± 0.8 | <.01 |
| Lymphocyte (%) | 22.9 ± 2.0 | 17.5 ± 2.4 | <.01 |
| Lymphocyte (×103/μL) | 1.4 ± 0.2 | 1.2 ± 0.2 | .07 |
Data are presented as mean and standard error (SE). WBCs, white blood cell.
Changes in T-cell subtypes populations and balances
Changes in the populations and balances of T-cell subtypes are presented in Table 3. A significant increase was found in CTL populations (27.5 ± 2.1% to 30.2 ± 2.5%, p < .03) after ablation therapy. Populations of Treg (2.1 ± 0.3% to 1.9 ± 0.3%, p = .16), Th1 (7.6 ± 1.1% to 6.7 ± 0.9%, p = .12), and Th2 (0.8 ± 0.2% to 0.9 ± 0.3%, p = .41) remained unchanged. The CTL/Treg ratios (18.8 ± 3.7% to 21.6 ± 3.6%, p < .05) increased significantly after ablation therapy, whereas Th1/Th2 ratios (13.7 ± 3.0% to 17.2 ± 3.5%, p = .12) remained unchanged.
Table 3.
Changes in T-cell population and balance after thermal ablation.
| Baseline | After treatment | p value | |
|---|---|---|---|
| T-cell subtypes/lymphocytes | |||
| CTL/lymphocyte (%) | 27.5 ± 2.1 | 30.2 ± 2.5 | <.03 |
| Treg/lymphocyte (%) | 2.1 ± 0.3 | 1.9 ± 0.3 | .16 |
| Th1/lymphocyte (%) | 7.6 ± 1.1 | 6.7 ± 0.9 | .12 |
| Th2/lymphocyte (%) | 0.8 ± 0.2 | 0.9 ± 0.3 | .41 |
| T-cell balance | |||
| CTL/Treg ratio | 18.8 ± 3.7 | 21.6 ± 3.6 | <.05 |
| Th1/Th2 ratio | 13.7 ± 3.0 | 17.2 ± 3.5 | .12 |
Data are presented as mean and standard error (SE).
CTL: cytotoxic T-lymphocyte; Treg: regulatory T-cell; Th1: Type-1 helper T-cell; Th2: Type-1 helper T-cell.
Sub-analysis of changes in CTL, Treg, and CTL/Treg ratio in each parameters
From the sub-analysis of T-cell balance changes, significant increases in CTL/Treg ratios were found in males (p < .02), metastatic tumor (p < .02), lung (p < .05) or soft tissue (p < .03) ablation, and heat-based ablation (p < .02) (Table 4).
Table 4.
Sub-analysis of changes in CTL, Treg, and CTL/Treg ratio in each parameter.
| Parameter | Patient no. | CTL | Treg | CTL/Treg ratio | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | After treatment | p Value | Baseline | After treatment | p Value | Baseline | After treatment | p Value | ||
| Age (years) | 8 | |||||||||
| ≦60 | 11 | 27.6 ± 2.7 | 31.2 ± 2.9 | .10 | 2.6 ± 0.5 | 2.4 ± 0.6 | .52 | 15.9 ± 5.3 | 19.2 ± 4.2 | .33 |
| >60 | 27.4 ± 3.2 | 29.5 ± 3.9 | .24 | 1.8 ± 0.3 | 1.6 ± 0.2 | .43 | 20.9 ± 5.2 | 23.3 ± 5.5 | .14 | |
| Gender | ||||||||||
| Male | 10 | 30.8 ± 2.9 | 34.7 ± 3.4 | .10 | 1.8 ± 0.3 | 1.5 ± 0.2 | .17 | 23.4 ± 5.5 | 28.2 ± 5.7 | <.02 |
| Female | 9 | 23.9 ± 2.8 | 25.2 ± 3.1 | .22 | 2.6 ± 0.5 | 2.5 ± 0.6 | .78 | 13.7 ± 4.4 | 14.3 ± 2.8 | .82 |
| Primary diagnosis | ||||||||||
| Colorectal | 8 | 22.6 ± 2.5 | 24.5 ± 3.5 | .43 | 2.4 ± 0.3 | 2.2 ± 0.4 | .54 | 11.6 ± 3.4 | 15.7 ± 4.3 | .06 |
| Soft tissue | 8 | 28.5 ± 2.4 | 32.1 ± 2.9 | .12 | 2.1 ± 0.5 | 2.0 ± 0.5 | .75 | 19.4 ± 4.5 | 20.3 ± 2.8 | .79 |
| Lung | 2 | … | … | … | … | … | … | … | … | … |
| Gynecologic | 1 | … | … | … | … | … | … | … | … | … |
| Target tumor | ||||||||||
| Primary | 3 | 27.5 ± 2.1 | 30.2 ± 2.5 | .21 | 2.1 ± 0.3 | 1.9 ± 0.3 | .11 | 18.8 ± 3.7 | 21.6 ± 3.6 | .54 |
| Metastasis | 16 | 25.9 ± 2.3 | 28.5 ± 2.8 | .09 | 2.1 ± 0.2 | 1.8 ± 0.2 | .21 | 16.7 ± 3.7 | 20.7 ± 3.9 | <.02 |
| Ablation site | ||||||||||
| Lung | 7 | 27.9 ± 4.6 | 32.0 ± 5.3 | .07 | 1.8 ± 0.3 | 1.9 ± 0.5 | .71 | 23.2 ± 7.2 | 28.1 ± 7.9 | <.05 |
| Soft tissue | 5 | 26.7 ± 2.7 | 31.1 ± 3.6 | .13 | 3.5 ± 0.5 | 2.6 ± 0.8 | .06 | 7.9 ± 0.8 | 14.4 ± 2.2 | <.03 |
| Liver | 4 | 25.7 ± 2.2 | 29.4 ± 5.8 | .51 | 1.9 ± 0.2 | 1.7 ± 0.3 | .60 | 13.9 ± 2.0 | 17.8 ± 4.4 | .26 |
| Bone | 3 | 31.3 ± 3.9 | 30.2 ± 4.0 | .20 | 1.0 ± 0.2 | 1.3 ± 0.2 | .11 | 32.0 ± 6.4 | 24.4 ± 2.5 | .19 |
| Target tumor number | ||||||||||
| Single | 16 | 27.1 ± 2.1 | 30.6 ± 2.9 | .05 | 2.2 ± 0.4 | 2.0 ± 0.4 | .39 | 18.3 ± 3.8 | 20.3 ± 3.5 | .35 |
| 2 or 3 | 3 | 28.4 ± 5.4 | 29.4 ± 5.3 | .59 | 2.1 ± 0.3 | 1.8 ± 0.6 | .59 | 19.9 ± 8.9 | 24.5 ± 8.9 | .12 |
| Maximum diameter of target tumor (cm) | ||||||||||
| ≦2 | 13 | 24.4 ± 1.9 | 28.4 ± 2.8 | .35 | 2.1 ± 0.3 | 1.9 ± 0.3 | .82 | 15.5 ± 3.1 | 19.5 ± 3.4 | .06 |
| >2 | 6 | 34.1 ± 4.6 | 34.2 ± 5.2 | .94 | 2.3 ± 0.7 | 2.0 ± 0.7 | .44 | 25.9 ± 9.4 | 26.1 ± 9.0 | .96 |
| Ablation technique | ||||||||||
| Heat-based ablation | 14 | 26.6 ± 2.7 | 29.0 ± 3.0 | .13 | 2.1 ± 0.3 | 1.9 ± 0.3 | .39 | 18.0 ± 4.4 | 21.6 ± 4.7 | <.02 |
| Cryoablation | 5 | 29.9 ± 3.2 | 33.7 ± 4.6 | .24 | 2.3 ± 0.8 | 2.1 ± 0.9 | .66 | 21.0 ± 7.0 | 21.5 ± 4.3 | .92 |
| Ablative zone area (cm2) | ||||||||||
| ≦10 | 11 | 25.5 ± 2.1 | 29.3 ± 3.3 | .08 | 2.4 ± 0.4 | 2.2 ± 0.4 | .32 | 14.5 ± 3.1 | 17.4 ± 3.3 | .09 |
| >10 | 8 | 30.2 ± 4.1 | 31.5 ± 4.1 | .38 | 4.5 ± 0.5 | 3.9 ± 0.6 | .65 | 24.8 ± 7.3 | 27.5 ± 7.0 | .43 |
Data are presented as mean and standard error (SE).
CTL: cytotoxic T-lymphocyte; Treg: regulatory T-cell.
Discussion
The results of our study verified the hypothesis that ablation therapy can change the peripheral blood T-cell balance, although the total number of peripheral blood lymphocytes does not change.
Recent evidence suggests that T-cells play an important role in regulating cancer growth (11–13). Various T-cells balance pro-tumor and anti-tumor responses in the tumor microenvironment as well as in the systemic circulation (11,20). Actually, CTL is known as the major effector cell for tumor elimination (21). However, Treg generally suppresses or down-regulates the induction and proliferation of CTL (10,22). Therefore, a balance shift of CTL/Treg ratio towards CTL dominance is a positive prognostic factor in ovary (15), gastric (17), and colorectal (18) cancer. This study found that the peripheral blood CTL population (< 0.03) and CTL/Treg ratio (< 0.05) increased significantly after heat-based ablation. This result might reflect that tumor antigen-specific CTLs were stimulated by ablation therapy. Den Brok et al. (5) reported that antigen-specific CTLs increased at ten days after radiofrequency ablation in tumor bearing mouse model. Nobuoka et al. (23) reported that the populations of circulating antigen-specific CTLs increased in five of the nine hepatocellular carcinoma patients after radiofrequency ablation. Given that increased CTL/Treg ratio is a positive prognostic factor, ablation therapy seems to enhance systemic anti-tumor immunity by altering the CTL/Treg balance.
It is noteworthy that the CTL/Treg ratio increased significantly after heat-based ablation (p < .02), although no significant change was found in the CTL/Treg ratio after cryoablation (p = .92). This difference might be attributable to the altered post-ablation cytokine environment. Erinjeri et al. (7) demonstrated that the increase of IL-10 after cryoablation (4.6-fold) was much higher than in heat-based ablation (1.7-fold). IL-10, which is regarded as an immunosuppressive cytokine that promotes Treg differentiation (10), also reduces the production of IL-2 and IFN-γ from Th1; it can indirectly inhibit CTL activation (10,24).Therefore, the elevation of IL-10 after cryoablation might inhibit the increase of CTL/Treg ratio. Our findings of the elevation of CTL/Treg after heat-based ablation (but not after cryoablation) suggest that heat-based ablation may be a better modality than cryoablation to enhance systemic anti-tumor immunity.
A significant increase of the CTL/Treg ratio after ablation therapy was found for male patients (p < .02), metastatic tumor (p < .02), lung (p < .05) or soft tissue (p < .03) ablation. On the other hand, ablative zone area and tumor size did not affect the CTL/Treg ratio. (#1–6) Although the impact of patient background and tumor characteristics on post-ablation immune response remain poorly understood, recent clinical and pre-clinical studies also suggest that proportions and responses of T-cell subtypes differ depending on gender (25), tumor type (26), and organs (27). Consequently, immune response after ablation therapy might also differ depending on patient background and tumor characteristics. Continued studies must be conducted with a more stratified patient group.
This study was hindered by several limitations. The small number of enrolled patients, high dropout rate of enrolled patients, and inhomogeneous patient and tumor backgrounds might be readily apparent limitations of this study. Second, although change in the peripheral blood T-cell balance after ablation was confirmed in this study, blood sampling was performed at one point. Third, T-cell changes in tumor microenvironments were not evaluated. Erös de Bethlenfalva-Hora et al. (6) reported that CD3 positive T-cells are augmented in the tumor microenvironment after RF ablation. Further studies evaluating serial T-cell balance changes in both the peripheral blood and tumor microenvironments must be conducted in the near future. Fourth, the antigen-specific T-cell response was not evaluated in this study. Therefore, it is not specified whether the increase of CTL population and CTL/Treg ratio observed reflected the antigen-specific T-cell response. Fifth, we only focused on T-cell subtypes, and other immune cells and cytokines were not evaluated. Therefore, the entire picture of immunological change triggered by thermal ablation was not unveiled (#1–11). Finally, we did not evaluate the relations between T-cell balance changes and clinical outcomes. Currently, a combination approach of ablation therapy and immune therapy has been attracting attention because tumor ablation might serve as the useful platform of immunization (4). Despite these limitations described above, the results of our study also proved the change in immune environment after in situ tumor ablation therapy. The results of our study might serve as a catalyst for additional investigation of combination approaches of ablation and immune therapy.
In conclusion, ablation therapy alters the T-cell balance by increasing the systemic CTL/Treg, ratio. Heat-based ablation might be a more effective approach than cryoablation to enhance systemic anti-tumor immunity.
Footnotes
Disclosure statement
The authors report no conflict of interest.
References
- 1.Liapi E, Geschwind JF. Transcatheter and ablative therapeutic approaches for solid malignancies. J Clin Oncol 2007;25:978–86. [DOI] [PubMed] [Google Scholar]
- 2.Chu KF, Dupuy DE. Thermal ablation of tumours: biological mechanisms and advances in therapy. Nat Rev Cancer 2014;14:199–208. [DOI] [PubMed] [Google Scholar]
- 3.Dromi SA, Walsh MP, Herby S, Traughber B, Xie J, Sharma KV, et al. Radiofrequency ablation induces antigen-presenting cell infiltration and amplification of weak tumor-induced immunity. Radiology 2009;251:58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.den Brok MH, Sutmuller RP, Nierkens S, Bennink EJ, Frielink C, Toonen LW, et al. Efficient loading of dendritic cells following cryo and radiofrequency ablation in combination with immune modulation induces anti-tumour immunity. Br J Cancer 2006;95:896–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.den Brok MH, Sutmuller RP, van der Voort R, Bennink EJ, Figdor CG, Ruers TJ, et al. In situ tumor ablation creates an antigen source for the generation of antitumor immunity. Cancer Res 2004;64:4024–9. [DOI] [PubMed] [Google Scholar]
- 6.Erös de Bethlenfalva-Hora C, Mertens JC, Piguet AC, Kettenbach J, Schmitt J, Terracciano L, et al. Radiofrequency ablation suppresses distant tumour growth in a novel rat model of multifocal hepatocellular carcinoma. Clin Sci (Lond) 2014;126:243–52. [DOI] [PubMed] [Google Scholar]
- 7.Erinjeri JP, Thomas CT, Samoilia A, Fleisher M, Gonen M, Sofocleous CT, et al. Image-guided thermal ablation of tumors increases the plasma level of interleukin-6 and interleukin-10. J Vasc Interv Radiol 2013;24:1105–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schell SR, Wessels FJ, Abouhamze A, Moldawer LL, Copeland EM 3rd,. Pro- and anti-inflammatory cytokine production after radiofrequency ablation of unresectable hepatic tumors. J Am Coll Surg 2002; 195:774–81. [DOI] [PubMed] [Google Scholar]
- 9.Diehl S, Rincón M. The two faces of IL-6 on Th1/Th2 differentiation. Mol Immunol 2002;39: 531–6. [DOI] [PubMed] [Google Scholar]
- 10.Adeegbe DO, Nishikawa H. Natural and induced T regulatory cells in cancer. Front Immunol 2013;4:190. doi: 10.3389/fimmu.2013.00190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zamarron BF, Chen W. Dual roles of immune cells and their factors in cancer development and progression. Int J Biol Sci 2011;7:651–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Disis ML. Immune regulation of cancer. J Clin Oncol 2010;28:4531–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Angell H, Galon J. From the immune contexture to the immunoscore: the role of prognostic and predictive immune markers in cancer. Curr Opin Immunol 2013;25:261–7. [DOI] [PubMed] [Google Scholar]
- 14.Han S, Zhang C, Li Q, Dong J, Liu Y, Huang Y, et al. Tumour-infiltrating CD4(+) and CD8(+) lymphocytes as predictors of clinical outcome in glioma. Br J Cancer 2014;110:2560–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Preston CC, Maurer MJ, Oberg AL, Visscher DW, Kalli KR, Hartmann LC, et al. The ratios of CD8+ T cells to CD4 + CD25 FOXP3+ and FOXP3− T cells correlate with poor clinical outcome in human serous ovarian cancer. PLoS One 2013;8:e80063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ito N, Nakamura H, Tanaka Y, Ohgi S. Lung carcinoma: analysis of T helper type 1 and 2 cells and T cytotoxic type 1 and 2 cells by intracellular cytokine detection with flow cytometry. Cancer 1999;85:2359–67. [PubMed] [Google Scholar]
- 17.Shen Z, Zhou S, Wang Y, Li RL, Zhong C, Liang C, et al. Higher intratumoral infiltrated Foxp3+ Treg numbers and Foxp3+/CD8+ ratio are associated with adverse prognosis in resectable gastric cancer. J Cancer Res Clin Oncol 2010;136:1585–95. [DOI] [PubMed] [Google Scholar]
- 18.Suzuki H, Chikazawa N, Tasaka T, Wada J, Yamasaki A, Kitaura Y, et al. Intratumoral CD8(+) T/FOXP3 (+)cell ratio is a predictive marker for survival in patients with colorectal cancer. Cancer Immunol Immunother 2010;59:653–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang X, Erinjeri JP, Jia X, Gonen M, Brown KT, Sofocleous CT, et al. Margin size is an independent predictor of local tumor progression after ablation of colon cancer liver metastases. Cardiovasc Intervent Radiol 2013;36:166–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DeNardo DG, Coussens LM. Inflammation and breast cancer. Balancing immune response: crosstalk between adaptive and innate immune cells during breast cancer progression. Breast Cancer Res 2007;9:212. doi: 10.1186/bcr1746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rousalova I, Krepela E. Granzyme B-induced apoptosis in cancer cells and its regulation. Int J Oncol 2010;37:1361–78. [DOI] [PubMed] [Google Scholar]
- 22.Nishikawa H, Sakaguchi S. Regulatory T cells in cancer immunotherapy. Curr Opin Immunol 2014;27:1–7. [DOI] [PubMed] [Google Scholar]
- 23.Nobuoka D, Motomura Y, Shirakawa H, Yoshikawa T, Kuronuma T, Takahashi M, et al. Radiofrequency ablation for hepatocellular carcinoma induces glypican-3 peptide-specific cytotoxic T lymphocytes. Int J Oncol 2012;40:63–70. [DOI] [PubMed] [Google Scholar]
- 24.Mocellin S, Marincola FM, Young HA. Interleukin-10 and the immune response against cancer: a counterpoint. J Leukoc Biol 2005;78:1043–51. [DOI] [PubMed] [Google Scholar]
- 25.Pollow DP, Uhrlaub J, Romero-Aleshire MJ, Sandberg K, Nikolich-Zugich J, Brooks HL, et al. Sex differences in T-lymphocyte tissue infiltration and development of angiotensin II hypertension. Hypertension 2014;64:384–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hadrup S, Donia M, Thor Straten P. Effector CD4 and CD8 T cells and their role in the tumor microenvironment. Cancer Microenviron 2013;6: 123–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang L, Lizzio EF, Gubina E, Chen T, Mostowski H, Kozlowski S. Organ-specific cytokine polarization induced by adoptive transfer of transgenic T cells. J Immunol 2002;169:5514–21. [DOI] [PubMed] [Google Scholar]


