Abstract
Identifying emotional states and explicitly putting them into words, known as affect labeling, reduces amygdala activation. Crucially, bilinguals do not only label emotions in their native language; they sometimes do it in their foreign language as well. However, one’s foreign languages are less emotional and more cognitively demanding than one’s native language. Because of these differences, it is unclear whether labeling emotions in a foreign language will also cause downregulation of affect. Here, 26 unbalanced bilinguals were scanned while labeling emotional faces either in their native or foreign languages. Results on affect labeling in a foreign language revealed that not only did it not reduce amygdala activation, but it also evoked higher activation than affect labeling in a native language. Overall, foreign language processing undermines affect labeling, and it suggests that the language in which people name their emotions has important consequences in how they experience them.
Supplementary Information
The online version contains supplementary material available at 10.1007/s42761-021-00039-9.
Keywords: Affect labeling, Foreign language, Bilingualism, Emotions
Introduction
In the first books of the series, Albus Dumbledore and Harry Potter were the only characters to call the villain “He who must not be named” by his proper name, Lord Voldemort. They considered that not using his name was counterproductive since it increased the fearful respect that people had for Voldemort. In essence, through directly labeling the cause of fear, they were trying to downregulate an emotional reaction. Unbeknownst to them, they were using implicit emotional regulatory labeling to reduce societal fear. Mounting evidence supports their intuitions. Labeling emotions, expressed in written form for instance, helps people cope with traumatic experiences months after putting their story into written text (Hemenover, 2003; Pennebaker, 1997).
Putting feelings into words during an emotional experience can also have immediate effects. Explicitly identifying the emotionality conveyed by a face, that is, affect labeling, downregulates arousal as indexed by amygdala activation (Hariri et al., 2000; Lieberman et al., 2007; McRae et al., 2010). Not only does it immediately help to reduce affect during the emotional situation but it also has long-term benefits. Patients who used more emotional words during exposure therapy showed less skin conductance reactivity when presented with the feared stimulus one week later (Kircanski et al., 2012; Tabibnia et al., 2008). Overall, affect labeling appears to be a successful strategy for downregulating negative emotional responses, both immediately and across time (see Torre & Lieberman, 2018 for a review).
All this previous research, however, has been conducted in participants’ native language. Today, a great deal of the world population speaks two or more languages on a daily basis (e.g., 53% of Europeans do so according to Eurobarometer, 2012). People do not only express their emotions and inner thoughts in their native language anymore. A question remains unanswered, then: does labeling emotions in a foreign language help downregulate affect to the same degree as in a native language? We explore this question in a population of unbalanced bilinguals who acquired their second (foreign) language later in life (>5 years old) in an artificial setting (classrooms) and have lower proficiency in comparison with their native language. We focused on this population for two reasons: first, for social implications, given that a great deal of people who fall into this category use a foreign language in a daily basis; and second, because the characteristics of foreign language processing can influence the outcome of affect labeling. Specifically, two aspects of foreign language might influence the classical downregulation outcome of affect labeling: foreign language processing is more cognitively demanding and less emotional than native language processing.
Foreign language processing, either through speech perception or production, is more cognitively taxing than native language processing. In production, transforming a concept into its lexical counterpart takes more time (Ivanova & Costa, 2008) and leads to more tip-of-the-tongue phenomena (Gollan & Acenas, 2004). Speech perception is also more costly in a foreign language: people read more slowly, making shorter saccades and more fixations (Cop et al., 2015). Processing a foreign language also activates areas related to cognitive control (Abutalebi, 2008; Branzi et al., 2016) and increases physiological measurements that index cognitive load, such as skin conductance (García-Palacios et al., 2018) and pupil dilation (Iacozza et al., 2017). In general, foreign language processing, be it passively through comprehension or actively in articulation, requires more time and produces a higher cognitive load than native language processing.
The fact that foreign language processing is more cognitively demanding is especially relevant for affect labeling since its success has been partially attributed to the involvement of cognitive regulatory regions. Activation of the right ventrolateral prefrontal cortex has been found to be inversely correlated with amygdala activation during affect labeling (Lieberman et al., 2007). Crucially, semantic judgments in a foreign language are associated with higher activation in the right inferior frontal gyrus (Chee et al., 2001). This increased activation during foreign language use might negatively affect the downregulation of the amygdala since the region is already being taxed by foreign language processing. According to this detrimental hypothesis then, affect labeling in a foreign language will fail to reduce amygdala activation.
Processing a foreign language is not only more cognitively demanding, but also less emotional. As any unbalanced bilingual knows, swearing in a foreign language does not feel as good—or as bad—as doing it in the native one. Evidence from self-reported questionnaires supports this claim: people find it easier to swear in a foreign language and do it more frequently than in a native language (Dewaele, 2004, 2010). Furthermore, there is a decrease in arousal when hearing swear words in a foreign language as indexed by skin conductance reactivity (Harris et al., 2003), a finding that extends to emotional words in general (Harris et al., 2003) and other measurements of arousal, such as pupil dilation (Iacozza et al., 2017; see Pavlenko, 2012 for a review). More specific to our interest, amygdala activation while reading positive passages in a foreign language is decreased in comparison with reading in the native language (Hsu et al., 2015)1.
This foreign language aloofness might help people create distance from the situation and reduce emotional reactivity. Previous research has found preliminary evidence for this in clinical settings. Freud already reported that certain patients switched to their foreign language when the exchange became too emotional for them to handle (Freud, 1918). This notion was later posited as the detachment effect, that is, the observation that people feel more detached when expressing their feelings in a foreign language (Marcos, 1976). In fact, people tend to talk more about embarrassing memories in their foreign language than in their native one (Bond & Lai, 1986). In the same vein, foreign language reduces the physiological arousal created by a potentially threatening situation. Specifically, people informed of the association between a stimulus and an electric shock in a foreign language show less conditioning than people instructed in their native language (García-Palacios et al., 2018). Because this general aloofness, foreign language processing might produce a greater reduction in affect even than that observed so far when labeling affect in a native language. We posit this as the regulatory enhancement hypothesis.
Here, we contrast the enhancement and detrimental hypotheses by asking participants to label emotional faces in their native and foreign languages while undergoing functional magnetic resonance imaging (fMRI). The enhancement hypothesis predicts a greater reduction in amygdala activation when labeling emotions in a foreign language, while the detrimental hypothesis predicts that amygdala activation will not be modulated by affect labeling in a foreign language.
Methods
Participants
Thirty participants from the Center for Brain and Cognition database were selected. Participants were unbalanced Spanish (native)-English (foreign) bilinguals who had not lived more than 12 months in an English-speaking country, had at least an intermediate level of English as certified by Cambridge English Qualifications, and learned English at school after the age of 5. Participants were asked to self-report their foreign language skills (reading, writing, speaking, and comprehension) on a scale from 1 (low proficiency) to 7 (high proficiency): M reading = 6.20, SD = 0.80; M writing = 5.40, SD = 1.24; M speaking = 5.57, SD = 1.33; M comprehension = 6.12, SD = 1.03.
Four participants were excluded: one due to incidental findings in MRI images and three due to excessive head movements (more than 2 mm/degrees in any of the six directions). After exclusions, a final sample of 26 participants was analyzed (M age = 21.85, SD = 2.33, 65% women). Before taking part in the experiment, participants gave written consent as approved by the local ethics committee CEIC Parc de Salut del Mar (Universitat Pompeu Fabra) and in accordance with the Declaration of Helsinki.
Procedure and design
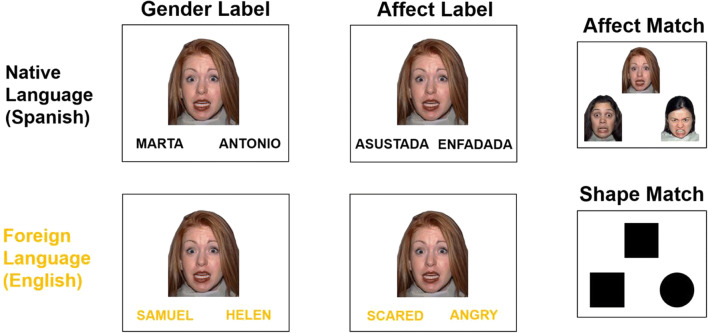
Participants were given detailed instructions of the task and did a practice trial before scanning. An affect labeling paradigm from previous literature was adapted for bilingual participants (Lieberman et al., 2007). During the task, participants saw a sample on the top of the screen and two options below, one to the left and another to the right of the screen (Fig. 1). In each trial, participants had to select which of the two stimuli, the right or the left one, matched the one on the top by pressing the corresponding remote control placed on their left or right hand. There were four matching rules: (1) Affect label: participants matched an emotional face to its corresponding emotional label (English: angry, scared, happy, and surprised; Spanish: enfadado/a, asustado/a, contento/a, and sorpendido/a), (2) Gender label: participants matched an emotional face to its corresponding gendered name, (3) Affect match: participants matched an emotional face to its corresponding emotional face, and (4) Shape match: participants matched geometric shapes (see Fig. 1).
Fig. 1.
Experimental design
Two versions were created for affective labeling and gender matching: one in the native language (Spanish) and another in their foreign language (English), leading to a total of six experimental conditions presented in a block design (see Fig. 1). Negative emotions were presented 80% of the time while positive emotions were only 20% of the emotional stimuli. Emotional faces were obtained from The NimStim Set of Facial Expressions (Tottenham et al., 2009).
At the beginning of each block, a sentence was presented for 2.5 s indicating which rule participants had to follow. Each block was compromised of 5 experimental trials (4 s each). After the experimental trials, a white screen with a black fixation dot was presented for 10 s. Overall, each block lasted 32.5 s and each condition was presented three times in a pseudorandomized order, for a total time of 9 min and 45 s.
Data acquisition
The fMRI data were collected using a Philips Ingenia 3-Tesla scanner. For each participant, we recorded 372 T2*-weighted gradient-echo echoplanar images (EPI, 46 slices, 3 × 3 × 3.1-mm resolution, no gap, interleaved order, matrix = 76 × 76, TR = 1600 ms, TE = 35 ms, flip angle = 70°). Slices were aligned parallel to the orbitofrontal cortex and covered the whole brain. In addition, a high-resolution T1-weighted image was acquired (190 sagittal slices, 1 mm thickness, RT = 9.8 ms, TE = 3.52 ms, flip angle = 8°, matrix = 240 × 240).
fMRI preprocessing
Image processing was carried out using SPM12 (Wellcome Trust Center for Neuroimaging, London, UK). Preprocessing functional scans included realignment to correct for motion-related artifacts, coregistration of the anatomical image to the mean functional image, segmentation of structural image, spatial normalization using parameters from the segmentation, and smoothing with an 8-mm (FWHM) Gaussian kernel.
Univariate analyses of sample-related activity
We used a general linear model as implemented in SPM12 to perform within subject fMRI analyses (Friston et al., 1994). The model included separate regressors for each of the six experimental conditions which were convolved with the canonical hemodynamic response function. The estimated head motion parameters were also included. Data were high-pass filtered (473 s) and temporal autocorrelation was controlled by an AR process. Contrast estimates were computed by subtracting the shape matching condition from the other conditions to control for the general effect of matching.
Statistical analyses
Given the hypotheses of the study, group analyses were focused on BOLD signal changes in amygdala ROIs. Based on previous research (Lieberman et al., 2007), amygdala ROIs were selected using the affect-matching condition as a functional localizer. The leave-one-subject-out cross validation method was used to avoid possible non-independence biases (Esterman et al., 2010). Specifically, we iteratively left out one subject and then we performed a whole-brain one-sample t test for the affect match/shape match contrast at a threshold of p < 0.05 FWE corrected at cluster-level, using voxel-level primary threshold of p < 0.001 uncorrected (see supplementary results). The resulting spatial map of significant results was masked with the left and right amygdala templates from the Anatomical Automatic Labeling atlas (Tzourio-Mazoyer et al., 2002) to determine amygdala voxels responding to emotionally evocative stimuli. This mask was applied to the excluded participant in each iteration in order to obtain the averaged amygdala activity (left and right separately) for each contrast comparing the labeling condition with the shape match condition. Subsequent analyses were performed in R, v. 3.5.1. These analyses consisted of a two-way repeated measures ANOVAs including condition (affect labeling or gender labeling) and language (L1 or L2) as factors. Equivalent analyses performed in SPM12 to investigate whole brain voxel-wise main effects and interaction effects of the previously described ANOVA are reported in supplementary results.
Results
Behavioral results revealed no differences between language conditions. Participants were accurate to a similar degree when labeling gender and affect in a native and a foreign language (all ps > .05, see Table 1). Participants thus responded to the task to a similar level of accuracy in both languages. Regarding reaction times, no differences were observed between affect label in a native language and affect labeling in a foreign language (t(25) = 1.25, p = .22, Table 1). Although participants were equally accurate when labeling gender (Table 1), they took significantly longer times to identify gender with names in their foreign language than with names in their native language (t(25) = −3.56, p = .001, Table 1).
Table 1.
Behavioral results
| Behavior | Condition | |||||
|---|---|---|---|---|---|---|
| Language-based | Non-language based | |||||
| Native language | Foreign language | |||||
| Gender label | Affect label | Gender label | Affect label | Affect match | Shape match | |
| Accuracy | 96.92% (4.31%) | 90.80% (8.86%) | 97.70% (5.00%) | 92.82% (7.28%) | 90.77% (7.80%) | 99.23% (2.17%) |
| Reaction time (ms) | 1169.72** (248.57) | 1472.74 (225.28) | 1257.31** (241.02) | 1432.83 (232.07) | 1475.76 (220.53) | 703.97 (113.37) |
Note. Mean accuracy and RT split by condition. Standard deviation in parenthesis. Only corrected responses were used for reaction times. **p-value < 0.01
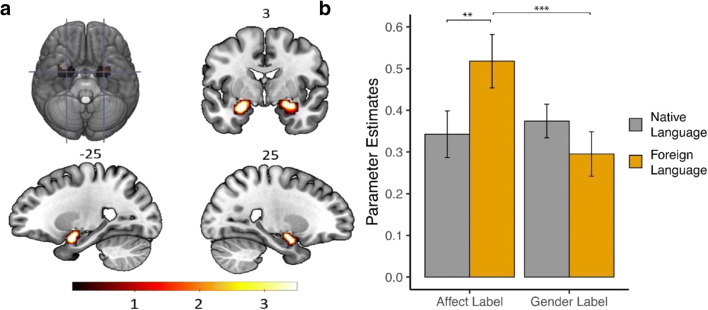
Following previous work (Lieberman et al., 2007), we analyzed BOLD signal changes in the amygdala ROI (Fig. 2A). Amygdala activation across conditions was compared with the shape match task. As expected, all conditions with emotional faces showed higher activation of the amygdala when contrasted with the shape match task (all ps < .001). We then ran two repeated measures ANOVA for the right and left amygdala with the type of matching task—affective or gender labeling—, and the language used—native or foreign—as factors in the model.
Fig. 2.
(A) The region of interest (ROI) was identified using the affect matching vs. shape matching contrast as a localizer. Illustration depicts significant voxels within the amygdala using the whole sample. Individual ROI masks were defined using the leave-one-subject-out cross validation method. The color bar represents the t-value applicable to the image and the values above slices are the MNI coordinate. (B) Parameter estimates of the averaged activity for the amygdala ROI are depicted for affect and gender labeling divided by language. Error bars represent ± standard error of the mean. **p-value < .01; ***p-value < .001
First, for the right amygdala, type of matching showed a significant main effect, F(1,25) = 6.05, p = .02, which was modulated by a significant interaction between type of matching and language, F(1,25) = 16.64, p < .001. No significant main effect was found for the left amygdala (all ps > .05), but the same interaction was close to significant as well, F(1,25) = 3.44, p = .07. Post hoc comparisons were run only for the right amygdala. Results revealed that activation was significantly higher for affect labeling than for gender labeling in a foreign language, t(25) = 4.87, < .001 (Fig. 2B), that is, the opposite of the result obtained in previous research conducted in a native language. The contrast between affect labeling in native and foreign language showed a similar pattern: labeling emotions in a foreign language produced greater amygdala activation than labeling emotions in a native language, t(25) = 2.96, p = .006 (see Fig. 2B). Previous research has shown that the amygdala habituates rapidly to negative stimuli in the affect labeling task (Preckel et al., 2019). One possible explanation for the higher amygdala activation in a foreign language is that foreign language processing interferes with the habituation of the amygdala. We designed the experiment with relatively short blocks to prevent this from happening. In fact, contrasting amygdala activation between the first and last affect label blocks indicated that amygdala did not habituate to the emotional faces throughout the experiment (all ps > .1).
Regarding the specific native language contrasts, the averaged values for amygdala activation were lower when participants labeled affect than when they labeled gender (Fig. 2B), following a similar trend observed in previous research (Lieberman et al., 2007). However, this time, the difference was not significant, t(25) = −.59, p = .56. Finally, when affect labeling was contrasted with affective matching, the original effect was replicated: affect labeling in a native language significantly reduced amygdala activation, t(25) = −2.27, p = .03. Importantly, this was not the case for affect labeling in a foreign language since the activation was not significantly different from affective matching, t(25) = 0.40, p = .69.
Discussion
If one could choose which language to express one’s feelings in a bilingual interaction, which language should they choose? If the goal is to reduce emotionality, according to the results we obtained, labeling emotions in a foreign language will fail to accomplish this regulative goal—in other words, it will not downregulate negative emotions. The native language seems to be the language in which people feel and, at the same time, regulate their emotions. We found that the amygdala activation was not different between affect labeling in a foreign language and affective matching. In contrast, amygdala activation during affect labeling in a native language was lower than during affective matching, replicating previous results (Lieberman et al., 2007). Furthermore, amygdala activation during affect labeling was higher in a foreign language than in a native language. Putting feelings into words in a foreign language does not help reduce arousal as indexed by amygdala activation.
Two opposing hypotheses were tested: the detrimental hypothesis, which stated that the higher cognitive demands of foreign language use would impair the downregulation of the amygdala, and the regulatory enhancement hypothesis, which stated that foreign language aloofness would cause more downregulation of the amygdala. Results supported the detrimental hypothesis. This casts doubts on the usefulness of foreign language in clinical settings to regulate emotional reactions, as has been previously suggested (García-Palacios et al., 2018; Morawetz et al., 2017). Future research should explore under which conditions a foreign language can be used in clinical settings. This might vary depending on a plethora of factors, such as the goal of the therapy or the foreign language proficiency of the patient. In addition, factors like age of acquisition or acculturation cause a foreign language to be more emotional and proficient (Harris et al., 2006) and, consequently, might affect the outcome of affect labeling.
Although we replicated the main finding that affect labeling in a native language does reduce amygdala activation (Hariri et al., 2000; Lieberman et al., 2007), we failed to replicate the effect when comparing affect labeling with a more restrictive condition: gender labeling (Lieberman et al., 2007). It is important to note, however, that the direction of the effect was the same, as amygdala activation was lower when identifying emotion than when identifying gender. Furthermore, by adding foreign language processing, the capacity to downregulate emotions even in the native language condition might have been affected. Although we cannot test such fatigue effects with the current design, further research should explore these as they have important implications: foreign language processing is not only failing to downregulate emotion, but it might also affect the capacity to do so when switching back to the native language.
Current results also shed light on the mechanisms that have been proposed to explain affect labeling (see Torre & Lieberman, 2018 for a review). Four different non-mutually exclusive theories exist: distraction, self-reflection, symbolic conversion, and reduction of uncertainty. The first mechanism, based on the idea that affect labeling distracts people from the emotional stimulus, will predict the opposite of the results we found since the distraction is arguably higher in a foreign language. The self-reflection mechanism is based on the idea that affect labeling reduces emotion because it promotes introspection. Although at first glance foreign language processing should promote the same degree of self-reflection than a native language, there is a way to reconcile our findings with this mechanism: foreign language processing might tax the cognitive system to such a degree that it blocks the introspection necessary for affect labeling to have an effect. Symbolic conversion, which states that downregulation emerges because of transforming stimuli into symbolic representation, is irreconcilable with the current findings since a foreign language transforms the stimulus into a symbol to the same degree as a native language, and yet, fails to reduce arousal.
Lastly, the final mechanism proposed, reduction of uncertainty, which falls under a constructionist theory of emotion, can potentially account for the current findings. The basis of this mechanism is that identifying emotions through labeling automatically reduces the uncertainty associated with the situation (Brooks et al., 2017; Lindquist et al., 2015). In fact, amygdala activation tracks different levels of uncertainty (Whalen, 2007)2. In order to reduce uncertainty, however, categorization needs to retrieve the sensory information and conceptual knowledge that is used to make meaning (Barrett, 2017; Lindquist et al., 2015). Both conditions are likely weakened in foreign language processing. First, sensory information is strongly imbedded into one’s native language. Second, semantic processing takes a longer time (Opitz & Degner, 2012) and has less representational strength in a foreign language (Strijkers et al., 2013). Our results provide the first evidence that the language in which people name emotions has important consequences in how they experience them. The way you label it, thus, matters for the way you feel it.
Supplementary Information
(DOCX 19 kb)
Author Contributions
M. L. V., V. C., C. A., and A. C. conceptualized and designed the experiment. M.L.V. and V.C. conducted the experiment and analyzed the results. M.L.V wrote the first version of the manuscript. V.C. and C.A. revised it.
Additional Information
Acknowledgements
We express our deepest gratitude to Albert Costa, mentor, colleague, and friend, who passed away in December 2018. We hope this manuscript does justice to his life’s work.
Funding
Víctor Costumero received funding from the Ministerio de Economía, Industria y Competitividad (IJCI-2016-29247) and the Ministerio de Ciencia, Innovación y Universidades (PID2019-105077RJ-I00). César Ávila received funding from the Spanish Government (PSI2016-78805-R), the Generalitat Valenciana (Aico/2018/038), and the Universitat Jaume I (UJI-B2018-22).
Open Practices Statement
Data for the main and supplementary results and behavioral task can be found in https://osf.io/qnj27/. Current experiment was not preregistered.
Conflict of Interest
The authors declare no competing interests.
Footnotes
Note that age of acquisition is an important factor since bilinguals who acquire their second language earlier in life and achieve great proficiency do not show disparity in emotional response between their languages (Harris, 2004).
The role or interpretation of the amygdala activation in the affect label task has been under dispute since shapes and faces differ in dimensions other than emotion (e.g., non-social vs. social). Recent research focused on disentangling which dimensions the amygdala is more sensitive to during affect labeling found that the amygdala does react especially to negative valence (or arousal), but habituates rapidly (Preckel et al., 2019). We opted for a design with shorter blocks to prevent habituation effects in the amygdala (Breiter et al., 1996), although the contrast used to define amygdala ROIs in our task does not allow us to completely dismiss the social dimension since it was not properly controlled.
References
- Abutalebi J. Neural aspects of second language representation and language control. Acta Psychologica. 2008;128:466–478. doi: 10.1016/j.actpsy.2008.03.014. [DOI] [PubMed] [Google Scholar]
- Barrett LF. The theory of constructed emotion: an active inference account of interoception and categorization. Social Cognitive and Affective Neuroscience. 2017;12:17. doi: 10.1093/scan/nsw154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond MH, Lai TM. Embarrassment and code-switching into a second language. Journal of Social Psychology. 1986;16:1543–1546. doi: 10.1109/Tasc.2005.869695. [DOI] [Google Scholar]
- Branzi FM, Della Rosa PA, Canini M, Costa A, Abutalebi J. Language control in bilinguals: monitoring and response selection. Cerebral Cortex. 2016;26:2367–2380. doi: 10.1093/cercor/bhv052. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Etcoff NL, Whalen PJ, Kennedy WA, Rauch SL, Buckner RL, Strauss MM, Hyman SE, Rosen BR. Response and habituation of the human amygdala during visual processing of facial expression. Neuron. 1996;17:875–887. doi: 10.1016/S0896-6273(00)80219-6. [DOI] [PubMed] [Google Scholar]
- Brooks JA, Shablack H, Gendron M, Satpute AB, Parrish MH, Lindquist KA. The role of language in the experience and perception of emotion: a neuroimaging meta-analysis. Social Cognitive and Affective Neuroscience. 2017;12:169. doi: 10.1093/scan/nsw121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chee MWL, Hon N, Lee HL, Soon CS. Relative language proficiency modulates BOLD signal change when bilinguals perform semantic judgments. NeuroImage. 2001;13:1155–1163. doi: 10.1006/nimg.2001.0781. [DOI] [PubMed] [Google Scholar]
- Cop U, Drieghe D, Duyck W. Eye movement patterns in natural reading: a comparison of monolingual and bilingual reading of a novel. PLoS ONE. 2015;10:e0134008. doi: 10.1371/journal.pone.0134008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewaele JM. The emotional force of swearwords and taboo words in the speech of multilinguals. Journal of Multilingual and Multicultural Development. 2004;25:204–222. doi: 10.1080/01434630408666529. [DOI] [Google Scholar]
- Dewaele JM. “Christ fucking shit merde!” Language preferences for swearing among maximally proficient multilinguals. Sociolinguistic Studies. 2010;4(3):595–614. doi: 10.1558/sols.v4i3.595. [DOI] [Google Scholar]
- Esterman M, Tamber-Rosenau BJ, Chiu YC, Yantis S. Avoiding non-independence in fMRI data analysis: leave one subject out. In NeuroImage. 2010;50:572–576. doi: 10.1016/j.neuroimage.2009.10.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freud S. From the history of an infantile neurosis. Standard Edition. 1918;17(7):124. [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline J-P, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: a general linear approach. Human Brain Mapping. 1994;2:189–210. doi: 10.1002/hbm.460020402. [DOI] [Google Scholar]
- García-Palacios A, Costa A, Castilla D, Del Río E, Casaponsa A, Duñabeitia JA. The effect of foreign language in fear acquisition. Scientific Reports. 2018;8:1157. doi: 10.1038/s41598-018-19352-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollan TH, Acenas LAR. What is a TOT? Cognate and translation effects on tip-of-the-tongue states in Spanish-English and Tagalog-English Bilinguals. Journal of Experimental Psychology: Learning Memory and Cognition. 2004;30:246–269. doi: 10.1037/0278-7393.30.1.246. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Bookheimer SY, Mazziotta JC. Modulating emotional responses: effects of a neocortical network on the limbic system. Neuroreport. 2000;11(1):43–48. doi: 10.1097/00001756-200001170-00009. [DOI] [PubMed] [Google Scholar]
- Harris CL. Bilingual speakers in the lab: psychophysiological measures of emotional reactivity. Journal of Multilingual and Multicultural Development. 2004;25:223–247. doi: 10.1080/01434630408666530. [DOI] [Google Scholar]
- Harris CL, Ayçiçeǧi A, Gleason JB. Taboo words and reprimands elicit greater autonomic reactivity in a first language than in a second language. Applied Psycholinguistics. 2003;24:561–579. doi: 10.1017/S0142716403000286. [DOI] [Google Scholar]
- Harris, C. L., Gleason, J. B., & Ayçiçeǧi, A. (2006). When is a first language more emotional? Psychophysiological evidence from bilingual speakers. In Bilingual minds: Emotional experience, expression, and representation. 10.1.1.69.5830
- Hemenover SH. The good, the bad, and the healthy: impacts of emotional disclosure of trauma on resilient self-concept and psychological distress. Personality and Social Psychology Bulletin. 2003;29:1236–1244. doi: 10.1177/0146167203255228. [DOI] [PubMed] [Google Scholar]
- Hsu CT, Jacobs AM, Conrad M. Can Harry Potter still put a spell on us in a second language? An fMRI study on reading emotion-laden literature in late bilinguals. Cortex. 2015;63:282–295. doi: 10.1016/j.cortex.2014.09.002. [DOI] [PubMed] [Google Scholar]
- Iacozza S, Costa A, Duñabeitia JA. What do your eyes reveal about your foreign language? Reading emotional sentences in a native and foreign language. PLoS ONE. 2017;12:e0186027. doi: 10.1371/journal.pone.0186027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanova I, Costa A. Does bilingualism hamper lexical access in speech production? Acta Psychologica. 2008;127:277–288. doi: 10.1016/j.actpsy.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Kircanski K, Lieberman MD, Craske MG. Feelings into words: contributions of language to exposure therapy. Psychological Science. 2012;23:1086–1091. doi: 10.1177/0956797612443830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman MD, Eisenberger NI, Crockett MJ, Tom SM, Pfeifer JH, Way BM. Putting feelings into words. Psychological Science. 2007;18:421–428. doi: 10.1111/j.1467-9280.2007.01916.x. [DOI] [PubMed] [Google Scholar]
- Lindquist KA, Satpute AB, Gendron M. Does language do more than communicate emotion? Current Directions in Psychological Science. 2015;24:99–108. doi: 10.1177/0963721414553440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcos LR. Bilinguals in psychotherapy: language as an emotional barrier. American Journal of Psychotherapy. 1976;30(4):552–560. doi: 10.1176/appi.psychotherapy.1976.30.4.552. [DOI] [PubMed] [Google Scholar]
- McRae K, Taitano EK, Lane RD. The effects of verbal labelling on psychophysiology: objective but not subjective emotion labelling reduces skin-conductance responses to briefly presented pictures. Cognition and Emotion. 2010;24:829–839. doi: 10.1080/02699930902797141. [DOI] [Google Scholar]
- Morawetz, C., Oganian, Y., Schlickeiser, U., Jacobs, A. M., & Heekeren, H. R. (2017). Second language use facilitates implicit emotion regulation via content labeling. Frontiers in Psychology, 8. 10.3389/fpsyg.2017.00366. [DOI] [PMC free article] [PubMed]
- Opitz B, Degner J. Emotionality in a second language: it’s a matter of time. Neuropsychologia. 2012;50(8):1961–1967. doi: 10.1016/j.neuropsychologia.2012.04.021. [DOI] [PubMed] [Google Scholar]
- Pavlenko A. Affective processing in bilingual speakers: disembodied cognition? International Journal of Psychology. 2012;47:405–428. doi: 10.1080/00207594.2012.743665. [DOI] [PubMed] [Google Scholar]
- Pennebaker JW. Writing about emotional experiences as a therapeutic process. Psychological Science. 1997;8:162–166. doi: 10.1111/j.1467-9280.1997.tb00403.x. [DOI] [Google Scholar]
- Preckel K, Trautwein FM, Paulus FM, Kirsch P, Krach S, Singer T, Kanske P. Neural mechanisms of affective matching across faces and scenes. Scientific Reports. 2019;9:1492. doi: 10.1038/s41598-018-37163-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strijkers K, Baus C, Runnqvist E, FitzPatrick I, Costa A. The temporal dynamics of first versus second language production. Brain and Language. 2013;127:6–11. doi: 10.1016/j.bandl.2013.07.008. [DOI] [PubMed] [Google Scholar]
- Tabibnia G, Lieberman MD, Craske MG. The lasting effect of words on feelings: words may facilitate exposure effects to threatening images. Emotion. 2008;8:307–317. doi: 10.1037/1528-3542.8.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torre JB, Lieberman MD. Putting feelings into words: affect labeling as implicit emotion regulation. In Emotion Review. 2018;10:116–124. doi: 10.1177/1754073917742706. [DOI] [Google Scholar]
- Tottenham N, Tanaka JW, Leon AC, McCarry T, Nurse M, Hare TA, Marcus DJ, Westerlund A, Casey BJ, Nelson C. The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry Research. 2009;168:242–249. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Whalen PJ. The uncertainty of it all. In Trends in Cognitive Sciences. 2007;11:499–500. doi: 10.1016/j.tics.2007.08.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 19 kb)