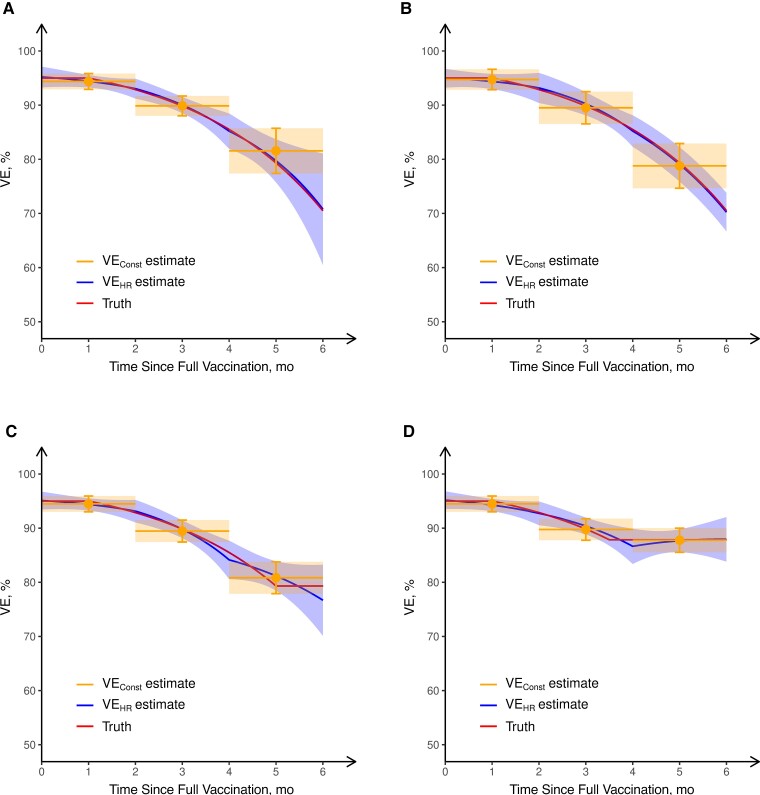
Figure 1.
Estimation of vaccine efficacy (VE) against symptomatic coronavirus disease 2019 ( COVID-19) based on 6 months of follow-up in 4 simulated clinical trials. In the first 2 trials, the true vaccine efficacy on the hazard rate (VEHR) (“truth”) decreases (linearly in the log hazard ratio) from a peak of 95% at full vaccination that lasts 1 month to 70% at 6 months after full vaccination. In the trial depicted in A, most participants received dose 2 at a calendar time coinciding with a peak in infection rates, whereas in the trial depicted in B, most participants received dose 2 at a time of low infection rates. In the trials depicted in C and D, the true VEHR plateaus at 5 and 3.5 months, respectively. In each trial, VEConst (VE estimate obtained under the standard Cox or Poisson model, assuming a constant VE over each time period), is obtained over 0–2, 2–4, and 4–6 months after full vaccination, and VEHR is estimated under the Cox model, in which the log hazard ratio is a piecewise linear function of time since vaccination, with change points at 0, 2, and 4 months after full vaccination. For each trial, the mean and standard deviation of each estimator over 1000 replicates are shown by the solid curve and shaded area, respectively.