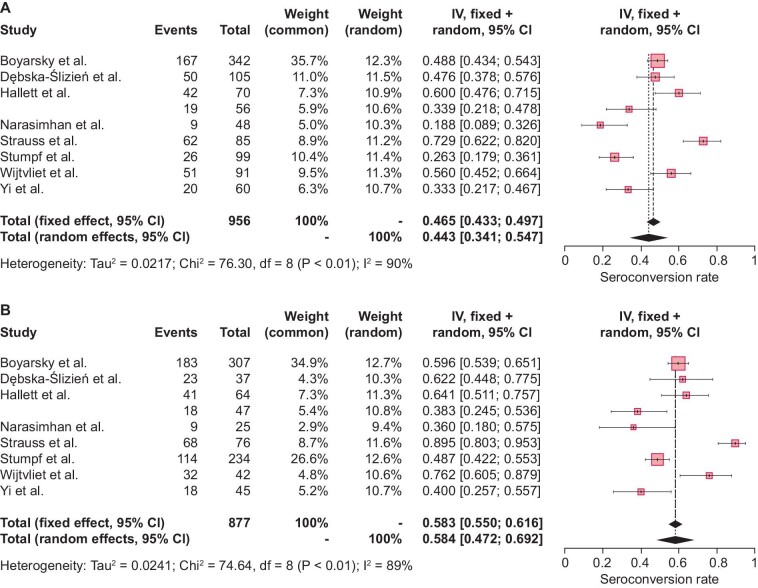
Figure 2:
Study-specific and pooled estimates for the seroconversion rate after two-dose mRNA vaccination with (A) BNT162b2 or (B) mRNA-1273 based on RE meta-analysis models and relying on the IV method. Box sizes in the forest plots are proportional to the weight assigned to each study. Limits of the displayed intervals are defined as 95% CIs. Eight studies calculated the seroconversion rates in SOT recipients after two-dose BNT162b2 vaccination (n = 956), (A) resulting in a pooled seroconversion rate of 44.3% (95% CI 34.1–54.7). The same eight studies also described seroconversion rates in SOT recipients after two-dose mRNA-1273 vaccination (n = 877), (B) resulting in a pooled seroconversion rate of 58.4% (95% CI 47.2–69.2). df, degrees of freedom; I2, inconsistency index.