Abstract
Viral evolution was evaluated in 47 immunocompromised patients treated with sotrovimab. Sequencing of SARS-CoV-2 following therapy was successful in 16. Mutations associated with sotrovimab resistance were documented in 6; viral replication continued after 30 days in 5. Combination antibody therapy may be required to avoid acquired resistance in immunocompromised patients.
Keywords: SARS-CoV-2, Omicron, sotrovimab, resistance, immunocompromised
Sotrovimab is a monoclonal antibody that neutralizes severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) by binding to a highly conserved epitope in the receptor-binding domain of sarbecoviruses. It was one of the few approved monoclonals that retained activity against the Omicron BA.1 variant of concern (VOC) [1]. While its activity against Omicron BA.2 is limited, emerging data suggest it may again be useful to treat the most recent Omicron subvariants BA.2.12.1, BA.4, and BA.5 [2, 3].
Immunocompromised patients are at an increased risk for a severe outcome of coronavirus disease 2019 (COVID-19) even after full vaccination against SARS-CoV-2. Indeed, the vaccination response is often reduced and can be completely absent in patients with combined T-cell and B-cell dysfunction [4, 5]. Furthermore, prolonged viral replication and evolution have been described in the immunocompromised host [6, 7]. Given the risk for a poor outcome, monoclonal antibody–based therapy is frequently used to treat these patients. Viral evolution toward resistance against these monoclonal antibodies can arise when viral replication is not sufficiently contained, and this risk may be more pronounced during antibody monotherapy. Recently, the selection of mutations in the spike protein of the Delta VOC in 4 of 100 patients treated with sotrovimab was reported, and all 4 were immunocompromised [8]. Specifically, mutations were found at positions 337 and 340, known to reduce susceptibility to sotrovimab [9]. We studied viral evolution in 47 immunocompromised patients treated with sotrovimab for an infection with the Omicron VOC.
METHODS
Sotrovimab became available on 26 January 2022 at the Erasmus University Medical Center, Rotterdam. It was used to treat immunocompromised patients infected with the SARS-CoV-2 Omicron VOC in the outpatient and inpatient settings. Before treatment, patients were screened for the presence of SARS-CoV-2 antibodies using the LIAISON SARS-CoV-2 TrimericS immunoglobulin G (IgG) assay (DiaSorin). A SARS-CoV-2 polymerase chain reaction (PCR) test was performed at baseline and weekly thereafter, and after discharge until the PCR cycle threshold (Ct) value was ≥30 [10]. Baseline and follow-up samples with a Ct value <30 were sequenced on the Nanopore platform. Successful sequencing was defined as at least 90% of the genome covered with at least 30 times coverage. Only descriptive statistics were used. The Erasmus University Medical Center Institutional Review Board approved the study.
RESULTS
Of the 47 patients treated, 24 (51%) were male, the median age was 63 years (interquartile range [IQR], 51–67), and 31 (66%) had undergone an organ transplantation. Seventeen patients (36%) received triple immunosuppressive therapy (mycophenolic acid, calcineurin inhibitors, and corticosteroids) as antirejection drugs after solid organ transplant; 10 patients (21%) received anti-CD20 agents and thus B cell–depleting therapy. Thirty-two of 47 (68%) patients were hospitalized for their COVID-19 infection and were treated with sotrovimab on the COVID ward. Information on IgG spike antibody titers in the 30 days preceding sotrovimab therapy was available for 36 patients. Spike antibodies were negative in 22 (61%) and very low (1–300 BAU/mL) in 9 (25%). These low or negative antibody titers were observed despite a history of at least 2 messenger RNA vaccinations in 30 of 36 (83%) patients and 3 vaccinations or more in 24 of 36 (66%) patients. See Supplementary Data 1 (Supplementary Table 1) for more details on the baseline characteristics.
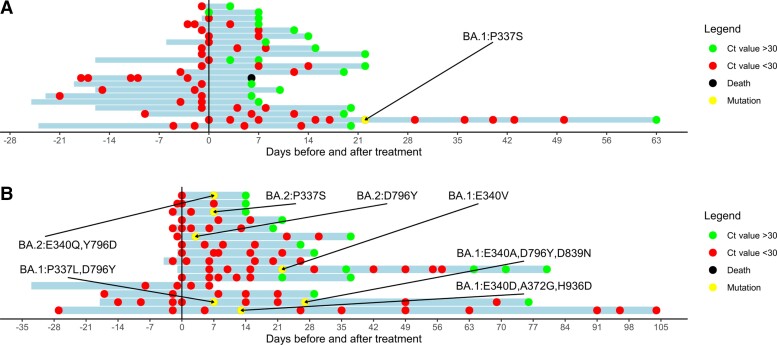
Sequencing was performed in 45 of the 47 patients; however, it was not successful in 14 (31%) patients due to low viral loads. Sequencing was not performed in 2 due to a negative PCR test in 1 patient and a very low viral load in the other. Sequencing before and after treatment with sotrovimab was successful in 16 patients. Furthermore, 10 patients only had a sequencing result prior to sotrovimab treatment, and 5 patients only had a sequencing result after sotrovimab treatment. Twenty-five patients were infected with the Omicron BA.1 subtype and 6 with the BA.2 subtype. Key spike mutations were detected on positions 337 and 340 (known to confer in vitro resistance to sotrovimab) in 6 of 16 (38%) patients with successful sequencing before and after sotrovimab (Figure 1). These mutations were found in 4 of 25 (16%) BA.1-infected patients and in 2 of the 7 (29%) infected with BA.2. In a third BA.2-infected patient, a D796Y mutation was found, but its impact on the neutralizing effect of sotrovimab is unknown. For more detailed information on the performance of sequencing and the characteristics of patients with spike mutations, see Supplementary Data 2 (Supplementary Tables 2 and 3) and Supplementary Figure 1.
Figure 1.
Follow-up of viral load in immunocompromised patients infected with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Omicron variant. Ct value of patients treated with sotrovimab with a baseline real-time polymerase chain reaction (RT-PCR) and at least 1 follow-up RT-PCR available. The y-axis indicates the day of sotrovimab infusion. The x-axis represents the time before/after sotrovimab infusion. Red dots represent a Ct value <30. Green dots represent a Ct value >30. Black dots represent the death of a patient. Yellow dots represent a sequence showing new spike mutations compared with the baseline sequence. Omicron sublineage and type of spike mutation are reported. The blue bars represent the period of follow-up, which started on the date of the first positive SARS-CoV-2 test and lasted until the last SARS-CoV-2 PCR at the Erasmus Medical Center (MC) or until death. In several patients, no dots are at the start of follow-up meaning that the first SARS-CoV-2 PCR test was not performed at Erasmus MC or that the test was performed on a device that did not report Ct values. A, Patients for whom not all pre- and post-treatment PCR sequencing was successful. B, Patients for whom all pre- and post-treatments PCR sequencing was successful. Five patients were lost to follow-up. Abbreviation: Ct, cycle threshold.
The median time to a Ct value ≥30 was 15 days (IQR, 8–22; range, 3–149). In contrast, the median time to a Ct value ≥30 in patients with a spike mutation was 50 days (IQR, 14–67). In 5 of 7 (71%) patients with spike mutations, low Ct values persisted 37, 63, 64, 76, and 149 days after treatment with sotrovimab (Figure 1).
DISCUSSION
Following treatment with sotrovimab, spike mutations associated with reduced in vitro susceptibility were detected in 6 of 47 patients overall and in 6 of 16 in whom sequencing was successful after therapy [9]. Furthermore, 4 patients infected with BA.1 and 1 patient infected with BA.2 continued to have a high viral load more than 4 weeks after treatment with sotrovimab. In all 4 patients who were infected with BA.1 and had a prolonged infection, mutations were found at position 337 or 340.
Our observations show that prolonged viral replication can be explained by treatment-related viral evolution toward resistance. This also illustrates that immunocompromised patients who are unable to clear SARS-CoV-2 despite antiviral therapy could serve as a source of new variants. These patients should be closely followed until viral clearance is documented whenever possible. Research is urgently needed to evaluate the value of direct-acting antivirals in this patient group. Similar to the treatment of human immunodeficiency virus, combination antiviral therapy might be required to reduce the risk for resistance. However, even when tixagevimab/cilgavimab combination therapy is used, only 1 of these antibodies retains in vitro activity against BA.2 and BA.4/5 variants. Therefore, monitoring treatment response with sequencing is recommended when available. An alternative treatment option may be very high titer convalescent plasma that was harvested from donors with a history of Omicron infection who also are fully vaccinated and boostered, considering the polyclonal nature of convalescent plasma [11, 12].
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Financial support. This work was supported by the European Union’s Horizon 2020, a research and innovation program of the European Union. Grants were received under the following projects: Versatile Emerging Infectious Disease Observatory (grant number 874735) and Rapid European SARS-CoV-2 Emergency Research Response (101003589). A grant from ZorgOnderzoek Nederland en het gebied Medische wetenschappen (ZonMw) (grant numbers 10150062010005 and 10430062010001) was also received.
Supplementary Material
Contributor Information
Sammy Huygens, Department of Internal Medicine, Section of Infectious Diseases and Department of Medical Microbiology and Infectious Diseases, Erasmus Medical Center, University Medical Center, Rotterdam, The Netherlands.
Bas Oude Munnink, Department of Viroscience, Erasmus Medical Center, University Medical Center, Rotterdam, The Netherlands.
Arvind Gharbharan, Department of Internal Medicine, Section of Infectious Diseases and Department of Medical Microbiology and Infectious Diseases, Erasmus Medical Center, University Medical Center, Rotterdam, The Netherlands.
Marion Koopmans, Department of Viroscience, Erasmus Medical Center, University Medical Center, Rotterdam, The Netherlands.
Bart Rijnders, Department of Internal Medicine, Section of Infectious Diseases and Department of Medical Microbiology and Infectious Diseases, Erasmus Medical Center, University Medical Center, Rotterdam, The Netherlands.
References
- 1. VanBlargan LA, Errico JM, Halfmann PJ, et al. . An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by therapeutic monoclonal antibodies. Nat Med 2022; 28:490–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Iketani S, Liu L, Guo Y, et al. . Antibody evasion properties of SARS-CoV-2 Omicron sublineages. Nature 2022; 604:553–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yamasoba D, Kosugi Y, Kimura I, et al. . Neutralisation sensitivity of SARS-CoV-2 omicron subvariants to therapeutic monoclonal antibodies. Lancet Infect Dis 2022; 22:942–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Haggenburg S, Lissenberg-Witte BI, van Binnendijk RS, et al. . Quantitative analysis of mRNA-1273 COVID-19 vaccination response in immunocompromised adult hematology patients. Blood Adv 2022; 6:1537–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sanders JSF, Bemelman FJ, Messchendorp AL, et al. . The RECOVAC Immune-Response study: the immunogenicity, tolerability, and safety of COVID-19 vaccination in patients with chronic kidney disease, on dialysis, or living with a kidney transplant. Transplant 2022; 106:821–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Corey L, Beyrer C, Cohen MS, Michael NL, Bedford T, Rolland M. SARS-CoV-2 variants in patients with immunosuppression. N Engl J Med 2021; 385:562–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Harari S, Tahor M, Rutsinsky N, et al. . Drivers of adaptive evolution during chronic SARS-CoV-2 infections. Nat Med 2022; 28:1501–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rockett R, Basile K, Maddocks S, et al. . Resistance mutations in SARS-CoV-2 Delta variant after sotrovimab use. N Engl J Med 2022; 386:1477–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cathcart AL, Havenar-Daughton C, Lempp FA, et al. . The dual function monoclonal antibodies VIR-7831 and VIR-7832 demonstrate potent in vitro and in vivo activity against SARS-CoV-2. BioRxiv 434607 [Preprint]. March 10, 2021 [cited 2022 Jul 06]. Available from: 10.1101/2021.03.09.434607. [DOI] [Google Scholar]
- 10. van Kampen JJA, van de Vijver DAMC, Fraaij PLA, et al. . Duration and key determinants of infectious virus shedding in hospitalized patients with coronavirus disease-2019 (COVID-19). Nat Commun 2021; 12:267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sullivan DJ, Gebo KA, Shoham S, et al. . Early outpatient treatment for COVID-19 with convalescent plasma. N Engl J Med 2022; 386:1700–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Huygens S, Hofsink Q, Nijhof IS, et al. Hyperimmune globulin for severely immunocompromised patients hospitalized with COVID-19: a randomized, controlled trial. J Infect Dis. In press. doi: 10.1093/infdis/jiac334. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.