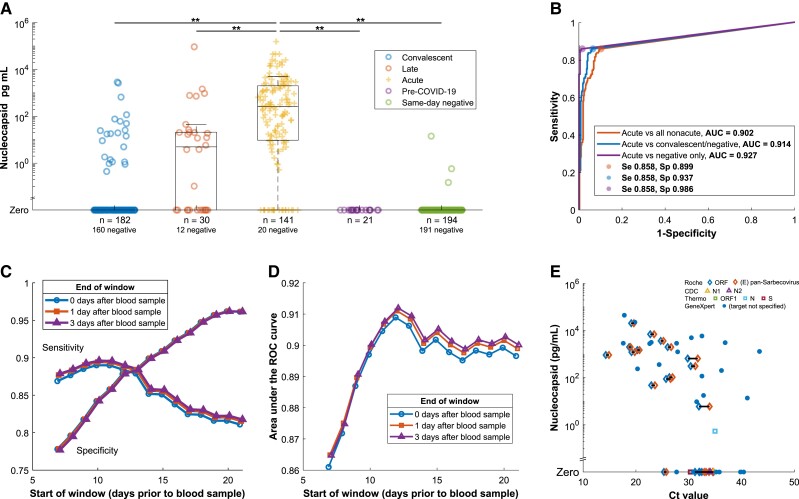
Figure 2.
A, Prevalence of antigenemia and serum or plasma nucleocapsid levels for blood samples by category. Unexpected results (presence of nucleocapsid in the convalescent and same-day negative groups, absence of nucleocapsid in the acute group) are examined in Supplementary Tables 2–5. B, ROC curve for diagnostic performance of detectable antigenemia with reference to a −14/+3 day window for acute infection. The additional curves progressively exclude ambiguous categories. C, Impact on sensitivity and specificity of varying the window period, which defines the reference standard for acute COVID-19. D, AUC for the same varied window periods. E, Antigenemia compared to RT-PCR Ct value for those specimens with a Ct value available from the clinical laboratory on the same day. Symbols correspond to assay and gene target with horizontal line linking Ct values for different targets detected in the same sample. This includes data from 4 assays on 3 thermocycler platforms described in further detail in the Supplementary material. Abbreviations: AUC, area under the curve; CDC, Centers for Disease Control and Prevention; COVID-19, coronavirus disease 2019; Ct, cycle threshold; N, nucleocapsid; ORF, open reading frame; ROC, receiver operating characteristic; RT-PCR, reverse transcription polymerase chain reaction; S, spike; Se, sensitivity; Sp, specificity.