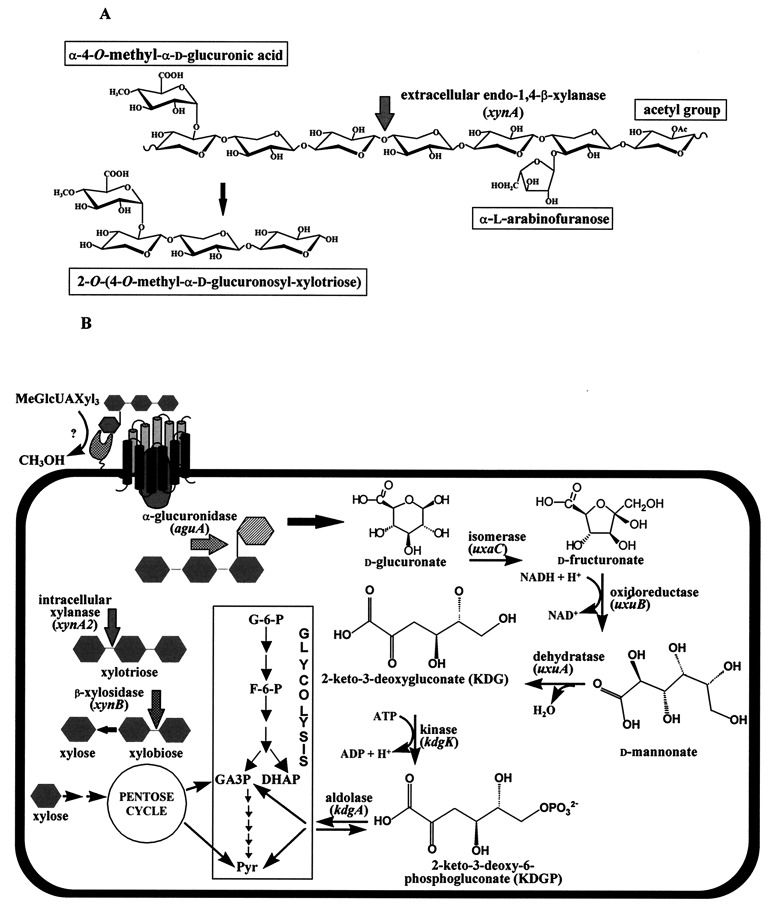
FIG. 1.
A proposed degradation pathway of MeGlcUAXyl3 in B. stearothermophilus T-6. (A) Xylan is composed of β-1,4-linked xylopyranose units which can be substituted with l-arabinofuranosyl, methyl-d-glucuronic acid, and acetyl side chains. The key enzyme in the degradation of xylan is an extracellular endo-1,4-β-xylanase (xynA). This enzyme releases short xylose units (xylobiose, xylotriose, and xylotetraose) which can be substituted with various side chains such as l-arabinose, d-glucuronic acid, or its 4-O-methyl ether, as in 2-O-α-(4-O-methyl-α-d-glucuronosyl)-xylotriose (MeGlcUAXyl3). (B) MeGlcUAXyl3 is demethylated and enters the cell via a specific transporting system. This system resembles the binding-protein-dependent transport systems in which a solute-binding lipoprotein interacts with integral membrane protein components that are involved in translocating the substrate across the membrane (22). Inside the cell, GlcUAXyl3 is cleaved by α-glucuronidase to yield xylotriose and d-glucuronic acid. The xylo-oligomers are hydrolyzed to xylose by intracellular xylanase and β-xylosidase. Xylose is converted into xylulose-5-phosphate, which can enter the pentose cycle. d-Glucuronic acid is converted into KDG by a three-step pathway catalyzed by uronate isomerase (uxaC), d-mannonate oxidoreductase (uxuB), and d-mannonate hydrolase (uxuA). KDG is then phosphorylated by KDG kinase (kdgK) to give KDGP, which is finally cleaved by KDGP aldolase (kdgA) to yield glyceraldehyde 3-phosphate (GA3P) and pyruvate. The latter two compounds can enter the Embden-Meyerhof-Parnas pathway.