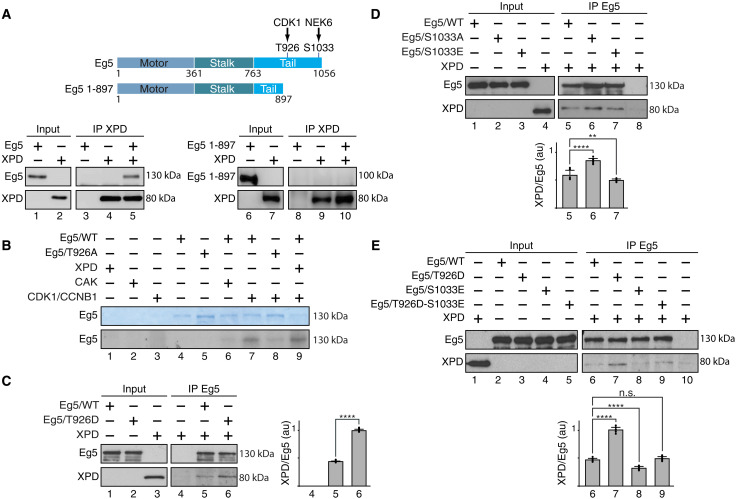
Fig. 2. Phosphorylation modulates the partnership of Eg5 with XPD.
(A) Schematic representation of the entire 1056–amino acid Eg5 protein (with the Motor, Stalk, and Tail domains) and the truncated form Eg5/1-897; the residues T926 and S1033 (which are phosphorylated by CDK1 and NEK6, respectively) are also indicated. Immunoprecipitated XPD (IP XPD) was incubated with either entire Eg5 or Eg5/1-897, and after washes, the coimmunoprecipitated proteins were resolved by SDS-PAGE and blotted with anti-XPD and anti-Eg5. (B) Purified Eg5/WT and Eg5/T926A were incubated (as indicated, +) with recombinant XPD, CAK (CDK7/cyclin H/MAT1), and CDK1/CCNB1 in the presence of [γ-32P]ATP (0.14 μM). Coomassie blue–stained gel (top) and the corresponding autoradiography (bottom) are shown. (C to E) When indicated (+), immunoprecipitated Eg5/WT, Eg5/T926D, Eg5/S1033A, Eg5/S1033E, or Eg5/T926D-S1033E was incubated with purified XPD/WT. After washes, the coimmunoprecipitated proteins were resolved by SDS-PAGE and blotted with anti-Eg5 and anti-XPD. The immunoprecipitated signals (IP) for XPD and Eg5 were quantified (n = 3, means ± SD), and the ratio XPD/Eg5 were plotted in arbitrary units (au). **P < 0.01 and ****P < 0.0001, Student’s t test; n.s., not significant.