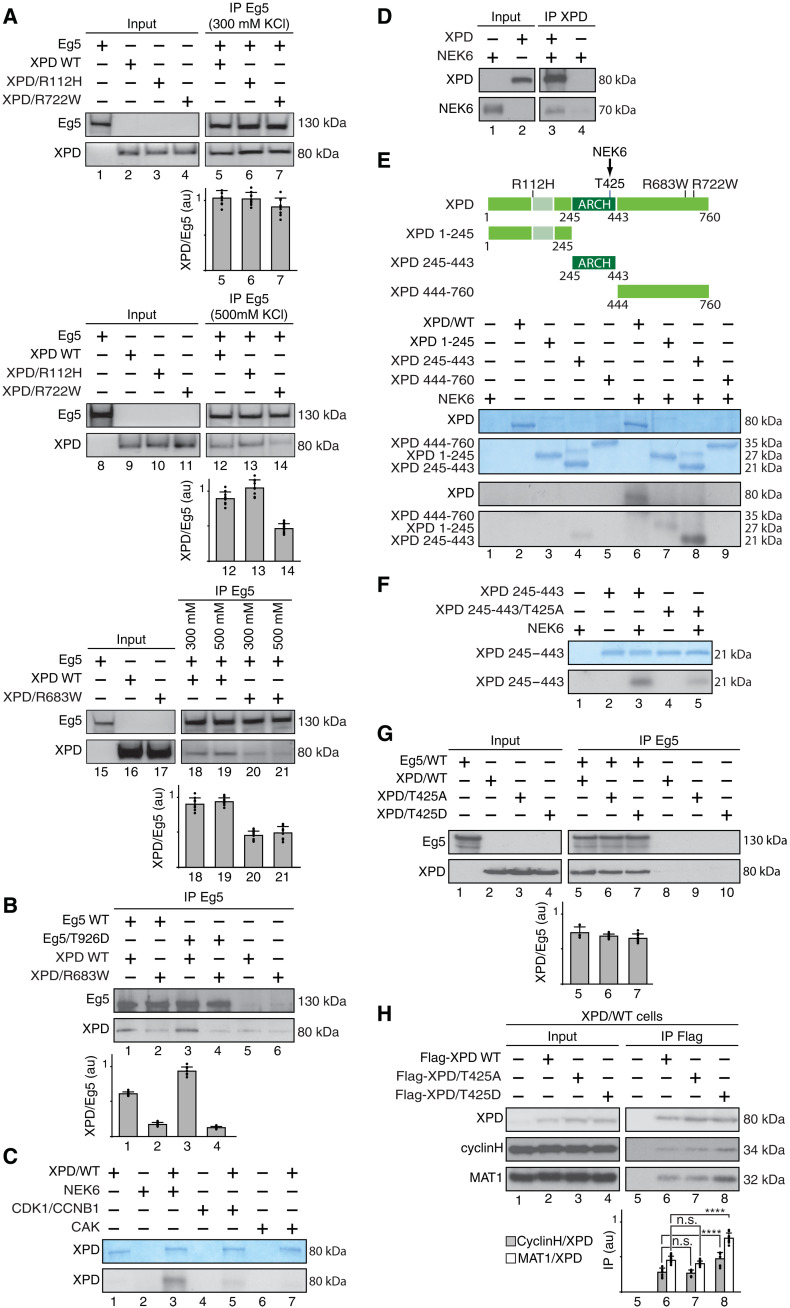
Fig. 3. Phosphorylation of Eg5 regulates its partnership with XPD.
(A) Coimmunoprecipitation at either 300 or 500 mM salt concentration of Eg5 (IP Eg5) with either XPD/WT, XPD/R112H, XPD/R722W, or XPD/R683W. The ratio XPD/Eg5 were plotted in au (n = 3, means ± SD). (B) Immunoprecipitated Eg5/WT and Eg5/T926D were incubated with either XPD/WT or XPD/R683W. The graph shows the ratio XPD/Eg5 (n = 3, means ± SD) in au. (C) Purified XPD was incubated with either recombinant NEK6, CDK1/CCNB1, or CAK (CDK7, cyclin H, and MAT1) in the presence of [γ-32P]ATP (0.14 μM). Coomassie blue–stained gel containing XPD (top) and the corresponding autoradiography (bottom) are shown. (D) Flag-XPD (IP XPD) was immunoprecipitated and incubated with tagged glutathione S-transferase (GST)–NEK6. As control, anti-Flag magnetic beads were incubated with GST-NEK6 alone. (E) Entire XPD and its truncated forms 1-245, 245-443, and 444-760 were incubated with NEK6 in the presence of [γ-32P]ATP (0.14 μM). Coomassie blue–stained gels and the corresponding autoradiographies are shown. (F) The ARCH domain (XPD 245-443) and its mutated form (XPD 245-443/T425A) were incubated with NEK6 in the presence of [γ-32P]ATP (0.14 μM). Coomassie blue–stained gel and the corresponding autoradiography are shown. (G) Coimmunoprecipitation of Eg5 (IP Eg5) with either XPD/WT, XPD/T425A, or XPD/T425D. The graph shows the ratio XPD/Eg5 (n = 3, means ± SD) in au. (H) Coimmunoprecipitation assays with whole-cell extracts isolated from XPD/WT cells overexpressing either Flag-XPD/WT, Flag-XPD/T425A, or Flag-XPD/T425D. The graph shows the ratio cyclin H/XPD (gray bars) and MAT1/XPD (open bars; n = 3, means ± SD) in au. ****P < 0.0001, Student’s t test.