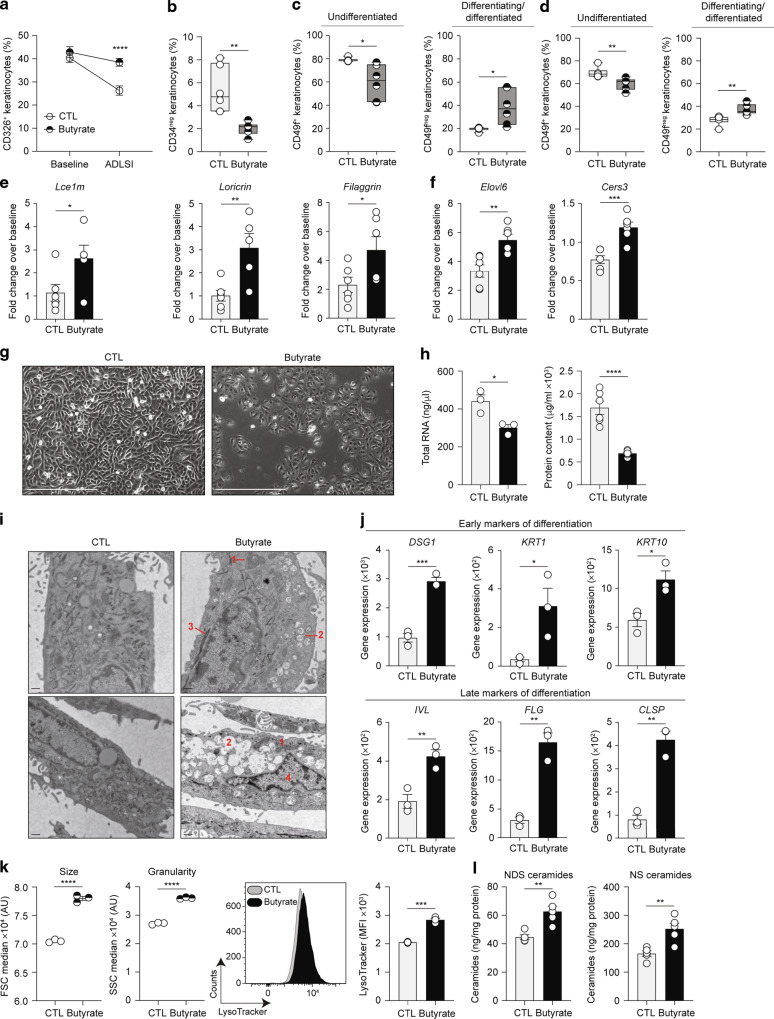
Fig. 5. Butyrate promotes terminal differentiation of epidermal keratinocytes.
a CD326+ keratinocytes in the skin of naive (“baseline”) and two-time house dust mite (HDM) allergen-sensitized (“ADLSI”) CTL and butyrate-exposed mice, as assessed by flow cytometry. b CD34+ hair bulge keratinocytes in HDM allergen-sensitized skin from water control (CTL) or butyrate-supplemented mice, as assessed by flow cytometry. CD49f+ basal CD326+ keratinocytes and CD49fneg differentiating/differentiated CD326+ keratinocytes in baseline (c) or two-time HDM allergen-sensitized (d) skin from CTL or butyrate-treated mice, as determined by flow cytometry. Fold-change expression over baseline of key components of the cornified envelope (e) or key enzymes for generation of long-chain fatty acids and ceramides (f) in fluorescence-activated cell sorting (FACS)-purified CD326+ CD34neg epidermal interfollicular keratinocytes isolated from two-time HDM allergen-sensitized control (CTL) or butyrate-treated mice, as determined by quantitative RT-PCR. Gene expression was normalized to β-actin. g Representative phase-contrast micrographs of primary human epidermal keratinocytes (HEK) either vehicle (CTL) or butyrate (500 μM)-supplemented for 48 h. Scale bars, 400 μm. h Total RNA and protein contents from HEK cultures. i Representative transmission electron microscopy micrographs of HEK cultures. Annotations: 1 = enlarged mitochondria; 2 = lysosomes; 3 = tonofibrils; 4 = degenerating nucleus. Scale bars, 500 nm. j Expression of skin barrier genes in HEK cultures, as determined by quantitative RT-PCR. Gene expression was normalized to β-ACTIN. k Median size (FSC, Forward scatter) and granularity (SSC, Side scatter) of HEK cultures, and assessment of the presence of acidic lysosome-like organelles by flow cytometry using LysoTracker probe. MFI, mean fluorescence intensity. l Quantification of ceramide production by HEK cultures, as determined by UPLC/ESI-MS/MS. Results are representative of data pooled from three (group “baseline”) and four (group “ADLSI”) independent experiments in a, from three independent experiments in b and d, from two independent experiments in c, e, and f, from at least three independent experiments in g, h, j, and k, and from one experiment in i and l. All results are expressed as mean ± SEM (n = 18 per group in “baseline” and n = 24 CTL and n = 23 Butyrate in “ADLSI” in a; n = 6 per group in b–e, and f; n = 3–6 per group in g, h, j, and k; n = 2 per group in i; n = 5 per group in l). Statistical significance was determined with Student’s t-test (unpaired, two-tailed) in a–f, h, and j–l. *P = 0.05, **P = 0.01, ***P = 0.001, and ****P = 0.0001. See also Fig. S3.