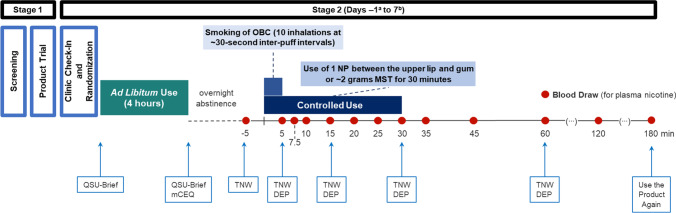
Fig. 1.
Study design. aThere was no controlled product use period on day − 1; participants completed the ad libitum use period followed by overnight abstinence on day − 1. The participants stayed in the clinic for a total of 8 days (day − 1 through day 7). bThere was no afternoon ad libitum use period after the controlled use period in the morning on the last study day, day 7. DEP, Direct Effects of Product Questionnaire; mCEQ, Modified Cigarette Evaluation Questionnaire; MST, moist smokeless tobacco; NP, nicotine pouch; OBC, own brand cigarette; QSU, Questionnaire on Smoking Urges; TNW, Tobacco/Nicotine Withdrawal Questionnaire