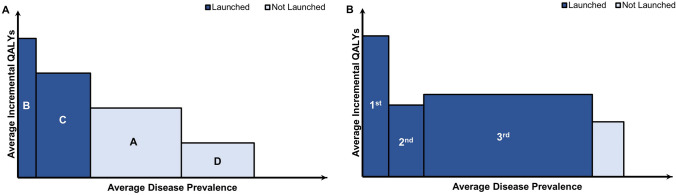
Fig. 1.
Indication launch decisions under single-price policies: A theory and B evidence. A shows the differential value and number of patients for a given drug per indication. Indication development follows the natural order A, B, C, then D - priced at then . Theory suggests that manufacturers may be incentivised to sequence and withhold indications according to clinical value and number of patients to extract the highest possible prices ( and ) under a single pricing mechanism [6, 7, 12]. B illustrates evidence from a sample of 25 multi-indication cancer drugs. This evidence suggests that launch sequences are indeed prioritized by number of patients (measured by disease prevalence [23]) and clinical value (measured by incremental QALYs extracted from health technology assessment reports). Indications offering marginal incremental QALYs for a small population group are not launched. QALY quality-adjusted life year. Source(s) [6, 7, 12, 23]