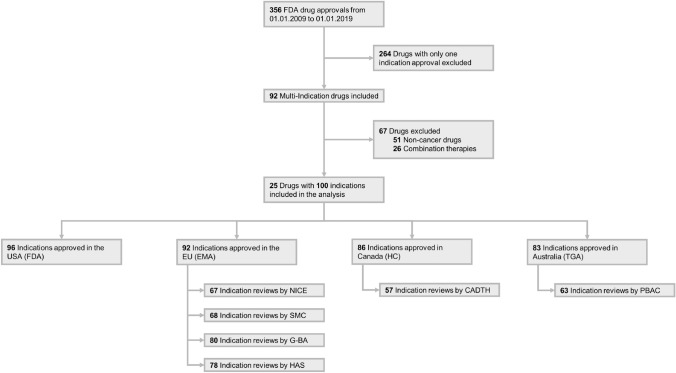
Fig. 2.
Flow diagram of multi-indication drugs included in the analysis, 2009–2019. CADTH Canadian Agency for Drugs and Technologies in Health, EMA European Medicines Agency, FDA US Food & Drug Administration, G-BA Federal joint Committee (“Gemeinsamer Bundesausschuss”), HAS Haute Autorité de Santé, HC Health Canada, NICE National Institute of Health and Care Excellence, PBAC Pharmaceutical Benefits Advisory Committee, SMC Scottish Medicines Consortium, TGA Therapeutic Goods Administration