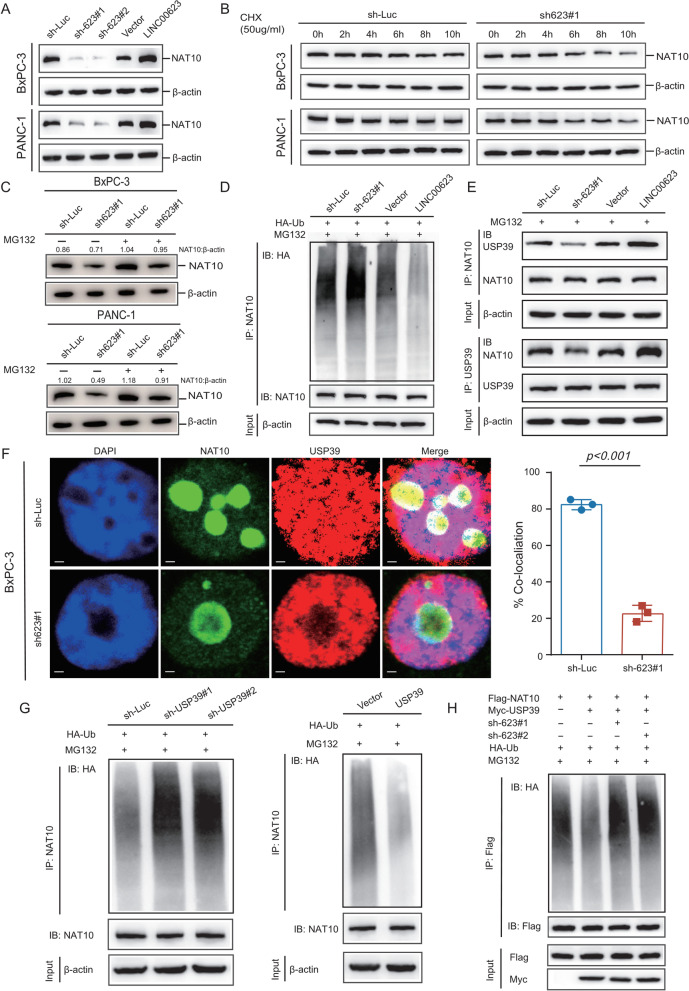
Fig. 4.
LINC00623 blocks NAT10 ubiquitination and degradation. a The protein levels of NAT10 in BxPC-3 and PANC-1 cells. β-Actin was used as the loading control. b The protein levels of NAT10 in the indicated cells treated with cycloheximide (CHX, 50 μg/mL) for 0, 2, 4, 6, 8, and 10 h. c The protein levels of NAT10 in the indicated cells treated with or without MG132 (10 μM) for 6 h. d BxPC-3 cell lysates were immunoprecipitated (IP) with an anti-NAT10 antibody and immunoblotted with an anti-HA antibody. e IP was performed to detect the association between NAT10 and USP39 in BxPC-3 cells. d–e BxPC-3 cells were transiently transfected with LINC00623 knockdown, LINC00623 overexpression or control plasmids and then treated with MG132 for 4 h before cell lysates were harvested for co-IP with an anti-NAT10 (IP: NAT10) or anti-USP39 (IP: USP39) antibody. f The colocalization of NAT10 (green) and USP39 (red) in BxPC-3 cells was evaluated by IF. Scale bar = 2 μm (left). The percentage of colocalization (Mander’s coefficients) of NAT10 and USP39 is summarized in the bar charts (right). g The ubiquitination levels of NAT10 were determined by Western blotting. BxPC-3 cells were transiently transfected with USP39 silencing (left), USP39 overexpression (right) and control plasmids and then treated with MG132 for 4 h before cell lysates were harvested for co-IP with an anti-NAT10 antibody and immunoblotted with an anti-HA antibody. h The ubiquitination levels of NAT10 in the products of IP with an anti-Flag antibody in lysates from BxPC-3 cells transfected with LINC00623 knockdown or USP39 overexpression and control plasmids