Fig. 6. miR-1290 and 1246 upregulate CNTF expression through reducing expression of FOXA2 in astrocytes.
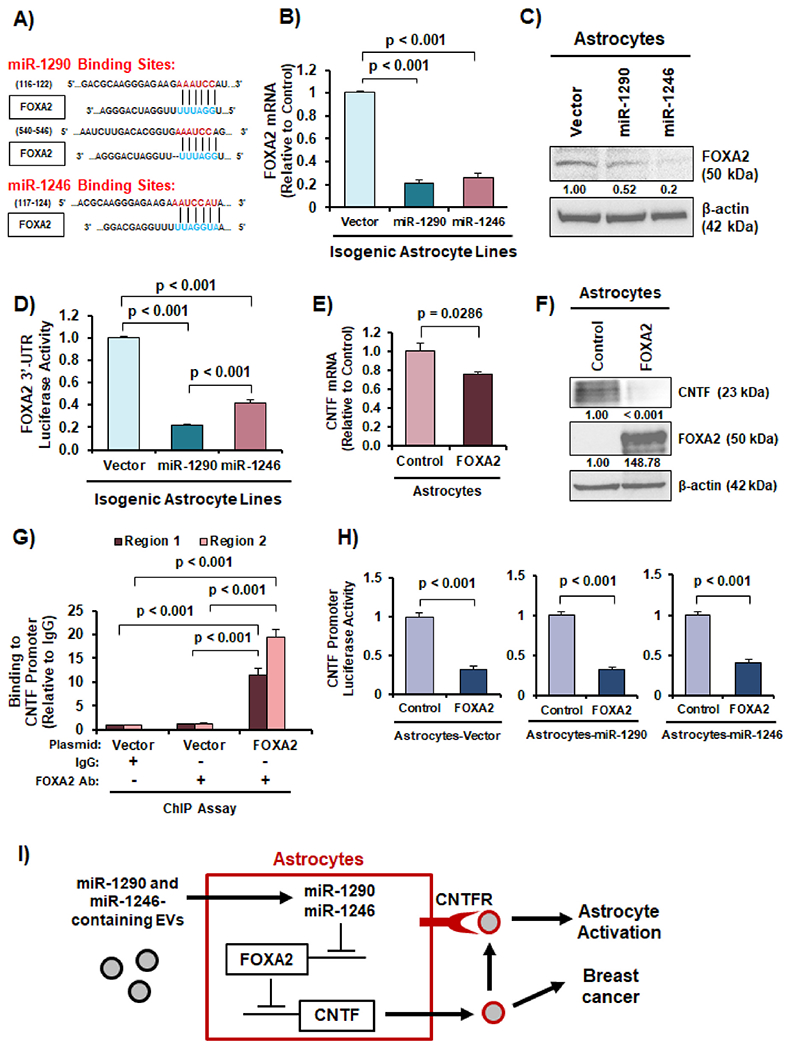
(A) Predicted miR-1290 and miR-1246 binding sites in the 3′-UTR of FOXA2 from Targetscan. (B) FOXA2 mRNA expression was reduced in astrocytes overexpressing miR-1290 and miR-1246. (C) FOXA2 protein expression was decreased in astrocytes transfected with miR-1290 and miR-1246 mimics as indicated by western blotting. (D) FOXA2 3′-UTR luciferase reporter activity was significantly lower in astrocytes overexpressing miR-1290 or miR-1246. (E) Ectopic expression of FOXA2 reduced CNTF mRNA expression in astrocytes as indicated by RT-qPCR. (F) Ectopic expression of FOXA2 decreases CNTF protein expression in astrocytes as indicated by western blotting. (G) FOXA2 binds to the human CNTF gene promoter in astrocytes as shown by ChIP-qPCR. (H) CNTF gene promoter activity was suppressed by miR-1290 or miR-1246 in astrocytes as indicated by dual luciferase reporter assay. (I) Schematic illustration of the novel EV-derived miR-1290/1246→FOXA2→CNTF signaling axis that leads to astrocyte activation and breast cancer aggressiveness. Students t-test was in Panels B, D-E, G-H to compute p-values. N = 3 experimental replicates.
