Figure 4. Comparison of SIV-infected cell recognition by miR-restricted RhCMV/SIV vector-elicited CD8+ T cells.
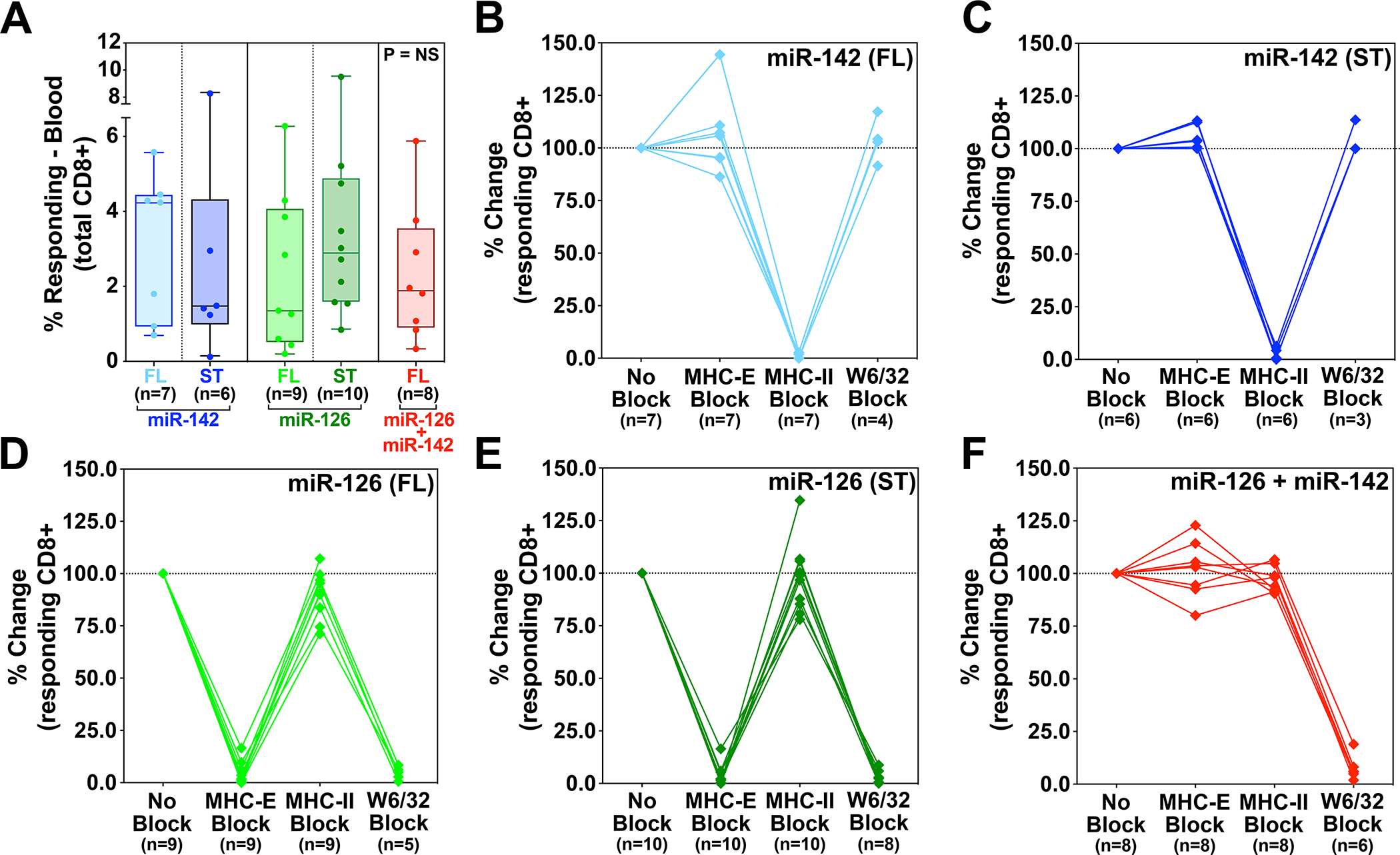
Analysis of autologous SIV-infected CD4+ T cell recognition by CD8β+ T cells isolated from RMs vaccinated twice with the miR-142-, miR-126-, or miR-142 + miR-126-restricted 68–1 RhCMV/SIV vectors, the former 2 vector types including independent analysis of both 3 vector sets expressing full length SIV Gag, Retanef, and 5’-Pol inserts and single supertope-focused vectors, whereas the miR-142 + miR-126-restricted RhCMV vector backbone was only studied with full length inserts. Plateau phase responses (>45 weeks after initial immunization) were defined by TNFα and/or IFN-γ production following peripheral blood-derived CD8β+ T cell incubation with autologous SIVmac239-infected versus mock-infected CD4+ T cells, with the MHC-restriction of the SIV-infected cell recognition determined by blocking analysis (see Fig. S10). (A) Analysis of the net SIV-infected cell-induced CD8+ T cell response frequency (subtracting mock-infected background) for RMs in each vaccine group. Kruskal-Wallis P-values comparing responses between all groups, and between groups with full length and supertope groups pooled, are shown in panel A. (B-F) Demonstration of the ability of anti-MHC-II mAb (MHC-II block), the MHC-E binding peptide VL9 (MHC-E block), or the pan-anti-MHC-I mAb (MHC-Ia and MHC-E block) to differentially inhibit SIV-infected cell recognition by CD8+ T cells in the different vaccine groups, with responses in the presence of blocking agents normalized to the unblocked response. Note that pan MHC-I blocking was not performed on all RMs due to cell number limitations. ST – supertope; FL – full length.
