Figure 7. Efficacy of miR-restricted RhCMV/SIV vectors.
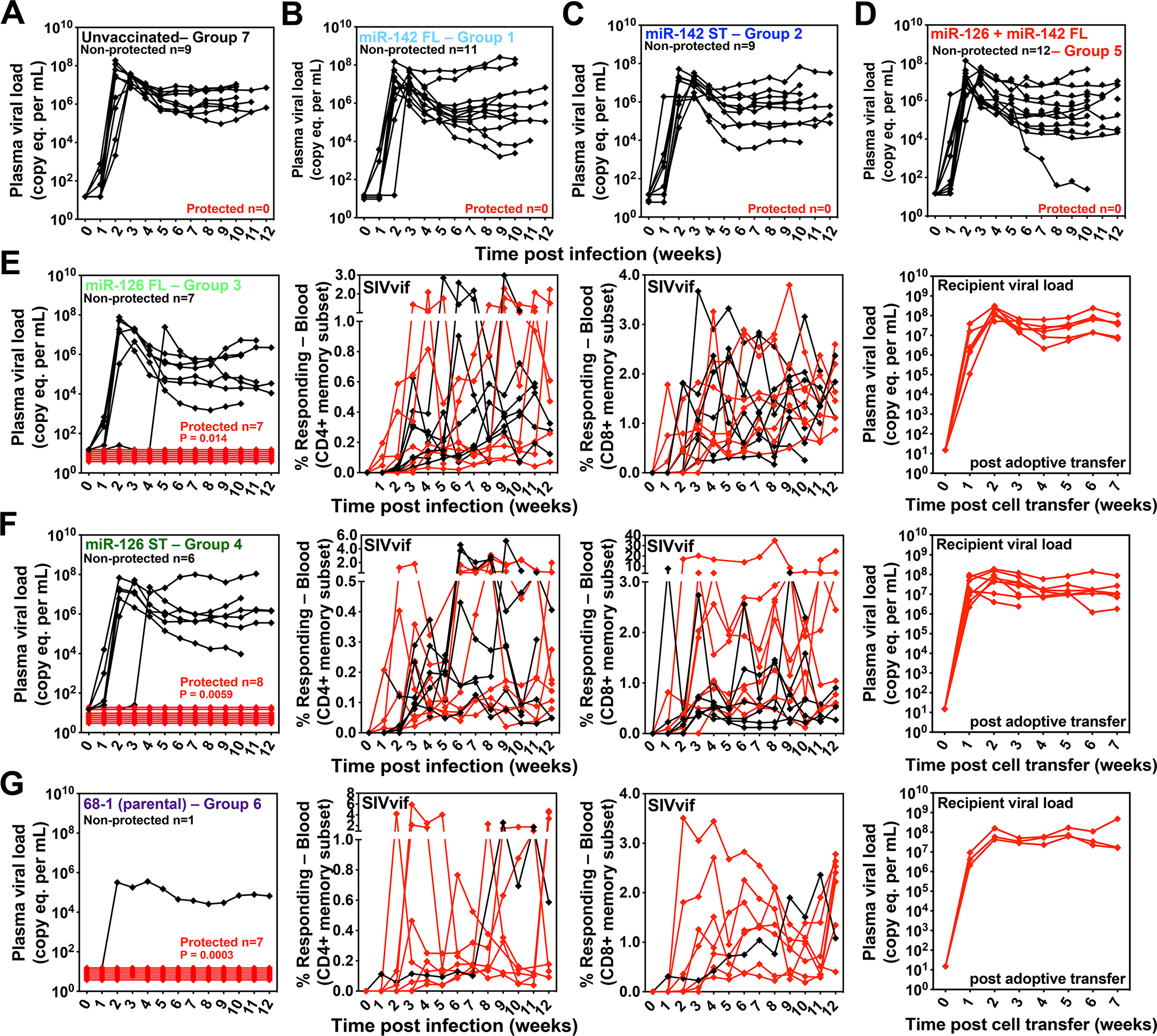
(A-G) Assessment of the outcome of SIV infection after repeated, limiting dose intra-vaginal SIVmac239 challenge of the designated vaccine groups (see Fig. 5A) by longitudinal analysis of plasma viral load (A-D) and/or de novo development of SIV Vif-specific CD4+ and CD8+ T cell responses (E-G). RMs were SIV challenged until the onset of sustained viremia and/or above-threshold SIV Vif-specific T cell responses, with the SIV dose administered 2 or 3 weeks prior to the initial response detection considered the infecting challenge (week 0). The overall n in each panel reflects the total number of RMs with such documented take of SIV infection during the challenge period. RMs with sustained viremia were considered non-protected (black); RMs with no or transient viremia but demonstrating sustained above-threshold SIV Vif-specific T cell responses were considered protected (red). Controlled SIV infection was confirmed in all 15 protected miR-126-restricted 68–1 RhCMV/SIV vector-protected RMs (both full length and supertope-focused inserts) and 3 of 7 parental 68–1 RhCMV/SIV vector-protected RMs by demonstration of sustained plasma SIV viremia in SIV-naïve recipient RMs after adoptive transfer of marrow and/or peripheral lymph node cells from the protected RMs, collected from between day 28 and 56 post-SIV infection (see table S6). Binomial exact P-values are shown where the proportion of protected RMs in a vaccine group differs significantly from the unvaccinated group. ST – supertope; FL – full length.
