Figure 8. Efficacy validation of miR-126-restricted (MHC-E-only) RhCMV/SIV vectors.
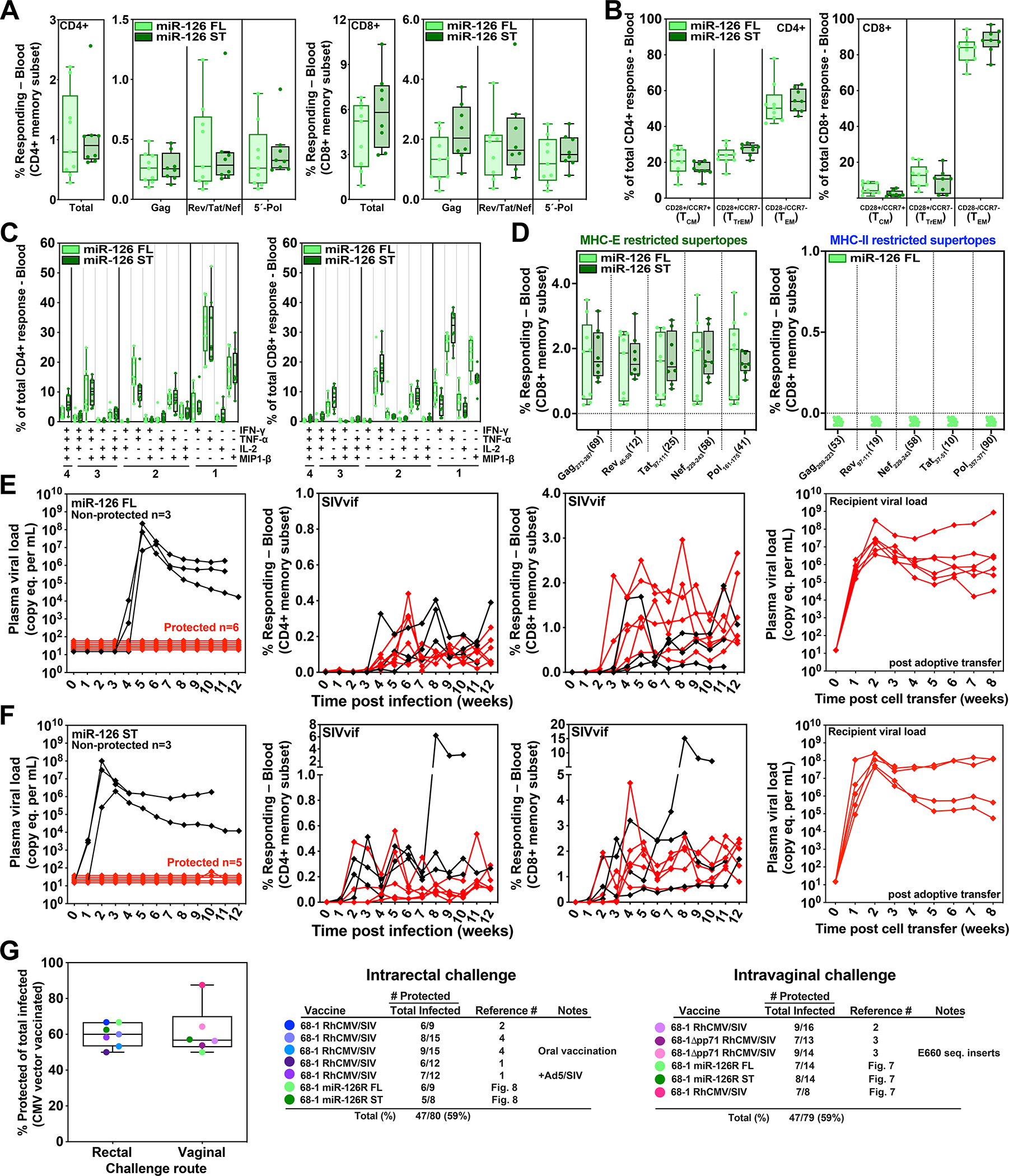
To confirm the efficacy of miR-126-restricted 68–1 RhCMV/SIV vectors after intra-rectal SIVmac239 challenge, we assembled a cohort of 17 RMs – including 9 and 8 RMs that were twice-vaccinated with the set of 3 full length insert expressing vectors or the single supertope-focused vector, respectively – for immunogenicity and efficacy analysis. (A-D) The plateau phase immunogenicity of these vectors, including response magnitude, memory phenotype and cytokine synthesis function, are shown, analyzed as described in Figs. 5 and 6. (E, F). RMs were subjected to repeated limiting dose intrarectal SIVmac239 challenge with outcome determined as described in Fig. 7. Controlled SIV infection was confirmed in all 11 miR-126-restricted 68–1 RhCMV/SIV vector-protected RMs by demonstration of sustained plasma SIV viremia in SIV-naïve recipient RMs after adoptive transfer (right panels) of cells collected from the protected RMs between day 28 and 56 post-SIV infection (see table S6). (G) Comparison summary of the efficacy of miR-126-restricted 68–1 RhCMV/SIV vectors from this study with all published cohorts of RMs vaccinated with non-miR-restricted 68–1 RhCMV/SIV-based vaccines, separated by challenge route. ST – supertope; FL – full length.
