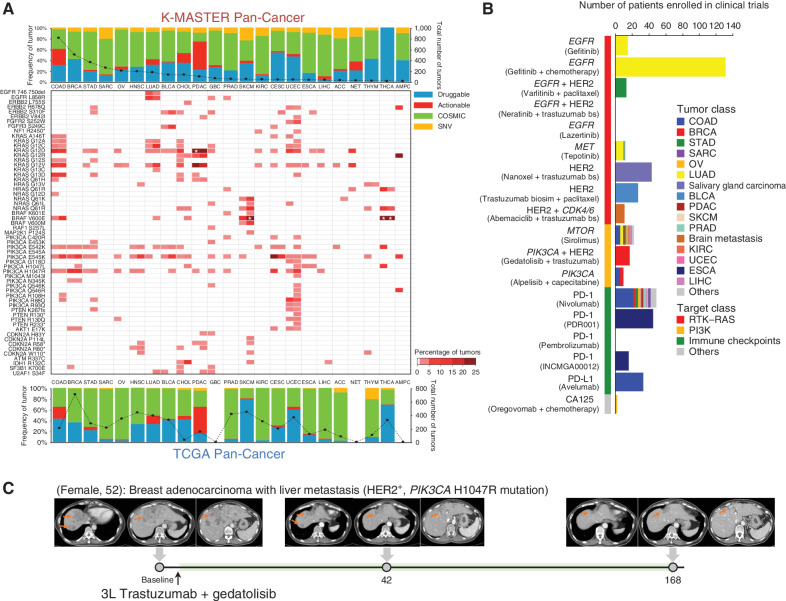
Figure 4.
Therapeutic landscape of pan-cancer patients based on ethnicity. A, The top bar graph depicts the distribution of major mutations based on the clinical actionability of the mutations in the K-MASTER cohort. The bottom bar graph represents the TCGA pan-cancer cohort. Mutations are categorized as “druggable” if they are FDA-approved biomarkers for FDA-approved drugs. Mutations are labeled as “actionable” if there is substantially compelling evidence to support the use of biomarkers to predict the response, especially the resistance of FDA-approved drugs. Mutations are labeled as “COSMIC” if they have been previously annotated using the COSMIC (Catalogue of Somatic Mutations in Cancer) database. Mutations that do not belong to any of the categories mentioned previously are depicted as “SNV.” Middle, frequencies of clinically actionable mutations across major cancer types in K-MASTER (left portion of the cell) compared with TCGA (right portion of the cell). Genes have been grouped by pathway. The white asterisk represents tumor types with more than 25% frequency (29.1% of KRASG12D mutation in K-MASTER PDAC, 43.6% of BRAFV600E mutation in TCGA SKCM, and 61.5% and 59.3% of BRAFV600E mutations in K-MASTER and TCGA THCA, respectively). B, The number of patients who have been enrolled to matched clinical trials based on unique molecular alterations. Trastuzumab bs, trastuzumab biosimilar. C, Clinical course of third-line (3L) trastuzumab and gedatolisib treatment in a BRCA patient with liver metastasis harboring a PIK3CA H1047R mutation. T1-weighted contrast-enhanced magnetic resonance images are shown for baseline, 6-week, and 24-week posttreatment. Orange arrows indicate measurable tumors. ACC, adrenal carcinoma; GBC, gallbladder cancer; NET, neuroendocrine carcinoma.