Fig. 2.
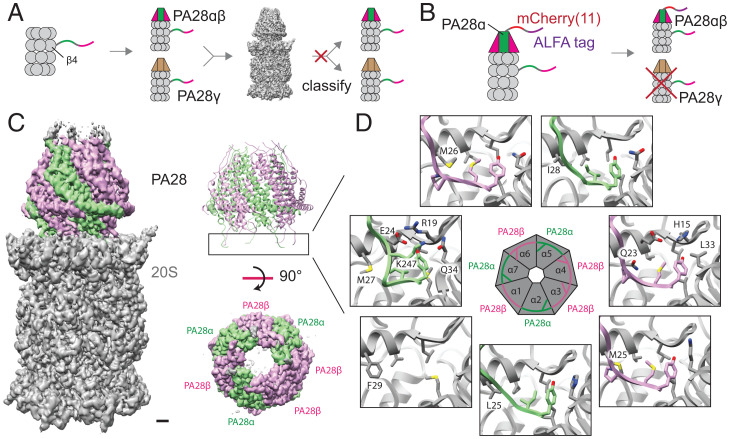
Structure and stoichiometry of the endogenous human PA28–20S proteasomal complex. (A) Tagging of the β4 subunit of the 20S proteasome yields both PA28αβ–20S and PA28γ–20S complexes, which cannot be computationally separated during cryo-EM image processing due to their high structural similarity. (B) Tagging of the PA28α subunit enables the purification of PA28αβ–20S without PA28γ–20S. (C) Cryo-EM map of PA28αβ–20S shows PA28 associating tightly with 20S, resulting in well-defined density for the entire complex. The high-quality density map allows clear assignment of three α and four β subunits in PA28 (SI Appendix, Fig. S5). (Scale bar, 10 Å.) (D) Six out of seven binding sites on the α-ring of 20S is occupied by the C-terminal tails of PA28 subunits.