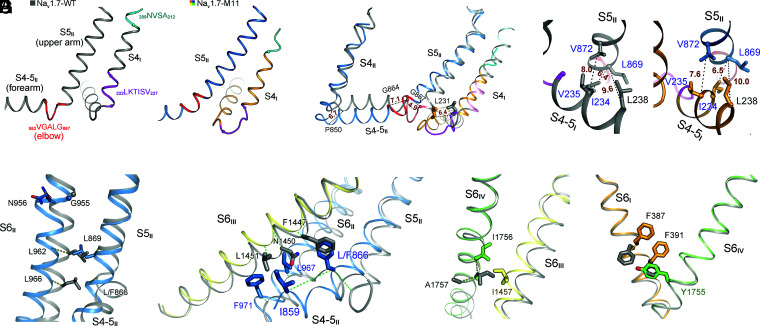
Fig. 5.
Electromechanical coupling of GC transfer to intracellular gating through concerted motions of adjacent segments. (A) Prominent secondary structure transition of the adjacent S4I and S4-5II segments. The corresponding sequences that undergo helical winding/unwinding between WT and M11 are highlighted with the same color in the two structures. Of note, the unwound sharp turn (elbow, colored red) between S4-5II and S5II in WT is transformed to a curved helical turn in M11. (B) Concerted structural shifts of S4-5I segments with the adjacent S4-5II and S5II segments. Structures of WT (gray) and M11 (domain colored) are superimposed as in Fig. 2A. Displacement of the Cα atoms of terminal residues on S4I, S4-5I, S4-5II, and S5II are indicated with dashed lines and labeled in ångstroms. (C) A hydrophobic cluster on the interface of S4-5I and S5II couples the concerted motions of these two segments. The distances between the indicated Cα atoms are labeled in ångstroms. (D) Concerted motions of S6II and S5II. The Cα atoms of the corresponding residues in the superimposed M11 and WT structures are connected by dashed lines to indicate their displacements. Leu962 and L966 on S6II would respectively clash with Leu869 and Leu866 (mutated to Phe in M11) on S5II had S6II not moved concertedly. (E) S6III is pushed inwardly by S4-5II and S6II. Displacements of the Cα atoms of the corresponding residues in M11 and WT are indicated by dashed lines. Representative residues in WT and M11 are shown to indict potential clashes should S6III not move accordingly. (F) Concerted motions of S6III and S6IV. S6IV undergoes an α→π transition that avoids clash between Ile1457 and Ile1756. (G) The subtle shift of S6IV leads to local change of S6I. Rotation of the aromatic rings of Phe387 and Phe391 seals the fenestration on the interface of repeats I and IV (Fig. 4D). It is noted that the conformational changes from WT to M11 depicted in F and G are nearly identical to those of Nav1.7 upon HWTX-IV binding to VSDII (26).