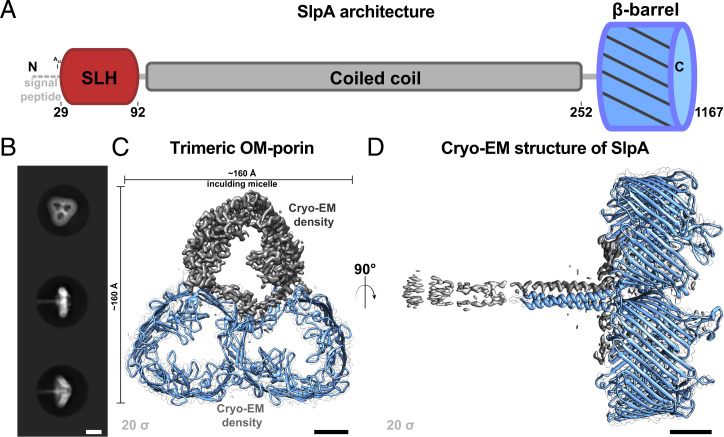
Fig. 1.
Cryo-EM reconstruction of D. radiodurans SlpA. (A) The SlpA protein contains a tripartite structure, including an N-terminal SLH domain, which is connected to a C-terminal β-barrel by a long coiled-coil segment. (B) Two-dimensional class averages of the trimeric SlpA specimen used for cryo-EM structure determination. Characteristic top and side views are shown. (C) Density map of the SlpA trimer (contour level on the Lower Left) shown from the Top. The resolution of the OMBB portion of the map is 2.9 Å, and resolution decreases toward the N-terminus, with a global resolution of 3.3 Å. Two subunits are shown as blue ribbons inside white envelope outlines and one as gray density (model hidden). Distance measurement includes the micelle density. (D) An orthogonal view of C, with the SlpA trimer shown from the side. The extended coiled coil degrades in resolution toward the N-terminus (see also SI Appendix, Fig. S1), presumably due to flexibility of the long stalk. (Scale bars in B, 100 Å; in C and D, 25 Å.)