Abstract
Tensin 2 (TNS2), a focal adhesion protein, is considered to anchor focal adhesion proteins to β integrin as an integrin adaptor protein and/or serve as a scaffold to facilitate the interactions of these proteins. In the kidney, TNS2 localizes to the basolateral surface of glomerular epithelial cells, i.e., podocytes. Loss of TNS2 leads to the development of glomerular basement membrane lesions and abnormal accumulation of extracellular matrix in maturing glomeruli during the early postnatal stages. It subsequently results in podocyte foot process effacement, eventually leading to glomerulosclerosis. Histopathological features of the affected glomeruli in the middle stage of the disease include expansion of the mesangial matrix without mesangial cell proliferation. In this review, we provide an overview of TNS2-deficient nephropathy and discuss the potential mechanism underlying this mechanosensitive nephropathy, which may be applicable to other glomerulonephropathies, such as CD151-deficient nephropathy and Alport syndrome. The onset of TNS2-deficient nephropathy strictly depends on the genetic background, indicating the presence of critical modifier genes. A better understanding of molecular mechanisms of mechanosensitive nephropathy may open new avenues for the management of patients with glomerulonephropathies.
Keywords: glomerulonephropathy, laminin α2, mechanosensitive protein, mechanotransduction, podocyte mechanics
Introduction
Tensin 2 (TNS2, also known as TENC1 or C1-TEN) and its family members, including tensin 1 (TNS1), tensin 3 (TNS3), and tensin 4 (TNS4), are focal adhesion proteins present in mammals [1, 2]. Focal adhesion proteins are multiprotein complexes consisting of the membrane receptors integrins as pivotal components. They connect the extracellular matrix (ECM) and cytoskeleton [3, 4]. Among focal adhesions, multiple reciprocal protein interactions (such as binding of cofactors or ligands, phosphorylation, and mechanical tension) orchestrate the signal transductions that regulate various cellular events, such as cytoskeletal rearrangement, cell proliferation, cell migration, cell death, cell differentiation, and gene expression [5,6,7,8,9]. A characteristic feature of the TNS protein structure is C-terminal tandem Src homology 2 (SH2)–phosphotyrosine-binding (PTB) domains, which allow TNS proteins to bind to membrane lipids, including phosphatidylinositol (3,4,5)-triphosphate (PIP3) [10,11,12], the cytoplasmic tails of β integrins [13,14,15,16], and other focal adhesion proteins, such as p130Cas (also known as BCAR1), focal adhesion kinase (FAK, also known as PTK2) [17, 18], integrin-linked kinase (ILK) [18], and deleted in liver cancer 1 (DLC1) [19,20,21,22]. These binding potentials indicate that TNS proteins can anchor focal adhesion proteins to β integrin as integrin adaptor proteins and/or serve as a scaffold to facilitate the interactions of these proteins. As expected, similar to other focal adhesion proteins, TNS deficits can perturb focal adhesion interactions or the regulation of integrin-mediated cellular physiology, such as adhesion, migration, and proliferation, and can have severe pathological effects [1, 2]. In the case of human diseases, genetic associations of focal adhesion genes have been most prevalently identified in cancers, followed by cardiovascular diseases [23]. For example, compared with healthy tissues, TNS2 was found to be downregulated in colorectal cancer (CRC) tissues [24]. Knockdown of TNS2 increased the proliferation and migration of CRC cell lines with high TNS2 expression, whereas overexpression of mouse Tns2 decreased the proliferation and migration of CRC cell lines with low TNS2 expression [25]. TNS2 deficiency was found to promote polyp formation in ApcMin/+ mice, a model of human familial adenomatous polyposis [25].
Overview of TNS2-deficient Nephropathy
In 2018, whole-exome sequencing and high-throughput exon sequencing for multiple cases with nephrotic syndrome revealed missense mutations in TNS2 as recessive causative mutations for nephropathy [26]. A Tns2 nonsense mutation (c.1546_1553del, p.Ser516Alafs*19, designated Tns2nph), which acts as the equivalent of a null allele, was identified as the recessive causative mutation for proteinuria in an ICGN mouse, a model of chronic kidney disease (CKD), by quantitative trait locus (QTL) analysis and subsequent nucleotide sequencing [27]. This genetic liability was evidenced by the phenotype of the genetically modified mouse: it carried a nonsense mutation in TNS2 that resulted in the partial loss of SH2–PTB domains [28].
TNS2 localization in the kidney
According to a microarray dataset of diverse cell types and tissues from adult mice (BioGPS accession number MOE430) [29], Tns2 mRNA is predominantly expressed in the lungs, followed by the heart, kidney, liver, adipose tissues, skeletal muscles, and diverse gland tissues. At the translational level, TNS2 is predominantly expressed in the glomerulus, according to the Human Protein Atlas (http://www.proteinatlas.org) [30]. The glomerulus, which is a spheroid capillary tuft located in the renal cortex, is the first segment of the nephron, where primary urine is produced by filtering the blood. It contains three cell types: glomerular endothelial cells, glomerular stromal cells (called mesangial cells), and glomerular epithelial cells (called podocytes). Podocytes are highly differentiated cells that have thick arms, major processes, and numerous subsequent projections or foot processes [31]. The glomerular basement membrane (GBM) is a specialized ECM that forms the capillary wall, while offering an epithelial basement membrane to podocytes (Fig. 1). The interdigitated foot processes of podocytes completely overlay the GBM and form membrane-like intercellular junctions, known as slit diaphragms [31]. In the glomeruli, TNS2 expression seems to be specific for podocytes (according to the Human Protein Atlas) [27, 32], but there is conflicting evidence regarding its expression in the mesangial cells [33]. TNS2 localizes to the basolateral surface of podocytes [32], where ECM receptors, including integrins, connect the GBM.
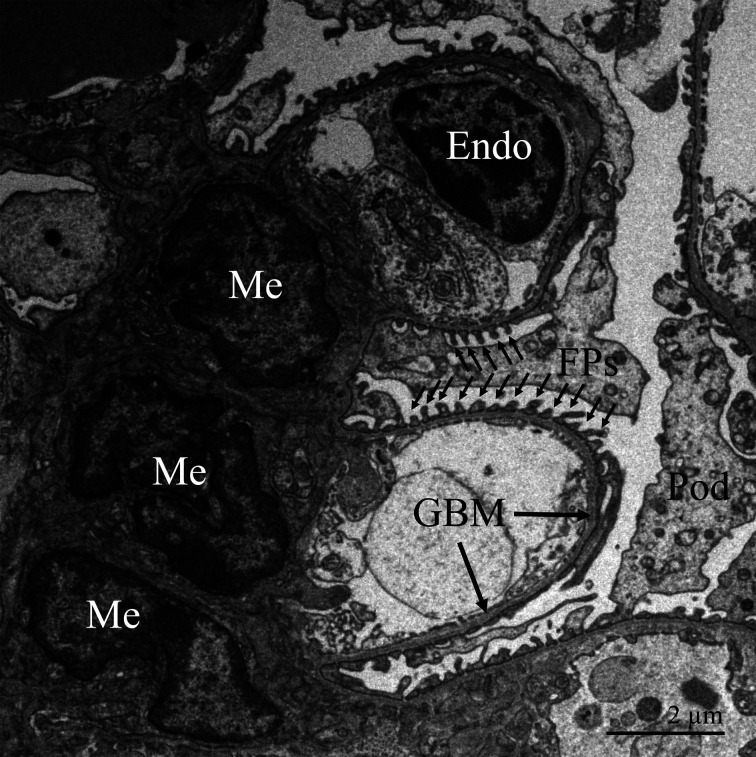
Fig. 1.
Transmission electron microscopic image of the glomerulus from an adult mouse. Glomerular endothelial cells (Endo) are specialized fenestrated cells that line the capillaries. The glomerular basement membrane (GBM) serves as a structural scaffold for the capillaries. Mesangial cells (Me) also support the capillaries. Podocytes (Pod) reside in Bowman’s space, and their interdigitated foot processes (FP) cover the epithelial side of the GBM.
Pathology
In Tns2-null mutant mice, no significant lesions have been reported in the perinatal kidney containing mature and immature glomeruli, the development stages of which can be morphologically classified as follows: S-shaped body, capillary loop, and maturing [32]. However, Tns2-null mutant glomeruli have been reported to form more numerous and larger GBM outpockets projecting toward the epithelial side in the maturation stage than wild-type glomeruli. These outpockets expand in the mutant glomeruli after birth, whereas they disappear in wild-type mature glomeruli [32, 34]. In the early postnatal stage, Tns2-null mutant mice have been reported to develop GBM lesions, forming a thick, multilayered pattern (known as lamellation) and a basket weave pattern (known as splitting), in addition to nonphysiological outpocketing [32, 34,35,36]. This abnormal ECM accumulation is followed by podocyte foot process alteration with the disappearance of the slit diaphragm, called effacement, which corresponds to a defect in glomerular filtration, resulting in proteinuria [34, 37, 38]. In contrast, glomerular endothelial cells exhibit normal development. Abnormal GBM materials are considered to be secreted from podocytes and not from endothelial cells [34]. Histopathological features of the affected glomeruli in the middle stage of the disease include expansion of the mesangial matrix without mesangial cell proliferation [35, 37, 39, 40]. This accumulation of mesangial matrix is thought to be caused by the phenotypic change in mesangial cells, which is accompanied by an increase in the expression of α-smooth muscle actin (α-SMA), and is slightly abrogated by prednisolone treatment [37]. The phenotypic change in mesangial cells may be induced by abnormal GBM materials because the formation of GBM lesions is followed by the appearance of α-SMA-positive mesangial cells. At the terminal stage, mesangial expansion progresses to the extent that it obliterates the glomerular capillaries, resulting in podocytopenia and glomerulosclerosis [35, 38]. After disruption of the glomerular filtration barrier, affected mice can develop tubulointerstitial injury, renal fibrosis, and eventually, end-stage renal disease, in accordance with the CKD progression pattern [40,41,42].
In humans, the severity of glomerular injury in patients with missense mutations in TNS2 probably varies according to the genetic background. In one study, three patients were diagnosed with minimal change nephrotic syndrome (MCNS), one was diagnosed with diffuse mesangial sclerosis (DMS), and one was diagnosed with focal segmental glomerulosclerosis (FSGS) [26]. This genetic susceptibility is discussed in a later section.
Potential Mechanism Underlying TNS2-deficient Nephropathy
The involvement of TNS2 in integrin signaling in podocytes is supported by its binding to integrin β1 in glomeruli [43] and by its localization in podocytes in vivo and in podocyte cell lines, which display a basolateral and peripheral dotted distribution pattern typical of a focal adhesion protein [26, 32, 44]. Furthermore, loss of the SH2–PTB domains in TNS2 impairs its ability to localize to focal adhesions and leads to the formation of glomerular lesions similar to those formed in Tns2-null mutant mice [44]. On the other hand, loss of the endogenous enzymatic activity of the protein tyrosine phosphatase (PTP) domain in TNS2, which catalyzes the dephosphorylation of phosphotyrosyl proteins, does not contribute to TNS2-deficient nephropathy [44]. These results indicate that the impairment of the TNS2-binding action of focal adhesions is the cause of TNS2-deficient nephropathy. As the binding potentials of the SH2–PTB domains indicate that TNS2 functions as an integrin adaptor protein and/or serves as a scaffold for other focal adhesion proteins, as mentioned above, an attractive hypothesis is that TNS2 deficiency perturbs multiple focal adhesion protein interactions that orchestrate the integrin signal transductions related to podocyte homeostasis. Podocyte-specific defects in either integrins (α3, β1) or integrin-binding molecules, such as CD151, ILK, and talin1, have been reported to result in nephrotic syndrome in murine models [45,46,47,48,49].
Adenovirus-mediated TNS2 overexpression also induces podocyte dysfunction in a manner that depends on PTP activity [50]. Overexpression of TNS2, which is observed in the glomeruli in diabetic nephropathy (DN) models, activates the mammalian target of rapamycin complex 1 (mTORC1) signaling pathway in podocytes through its PTP activity toward nephrin, a principal podocyte slit diaphragm component [50]. This mTORC1 activation is associated with podocyte dysfunction in DN [51, 52].
Mechanosensitive nephropathy
Interestingly, in Tns2-null mutant mice, the glomeruli located in the inner renal cortex have been reported to exhibit only mild GBM thickening, while those located in the outer renal cortex have been reported to exhibit rapidly progressive lesions after birth [32]. This difference has been attributed to the difference in developmental maturity of the glomeruli at birth. At birth, the glomeruli located in the outer renal cortex are still maturing, while those in the inner renal cortex are already mature without obvious lesions [32]. These findings indicate that TNS2 deficiency impacts podocyte development ex utero but not in utero. The biomechanical stress on podocytes substantially differs between these two conditions. Fetal kidneys are characterized by very low blood flow and high vascular resistance. After birth, glomerular hydraulic pressure rapidly rises because of a sharp decrease in renal vascular resistance, followed by an increase in intrarenal blood flow [53].
Cells can sense and respond to the biophysical properties of the extracellular milieu through integrin-based adhesion, including focal adhesion, via a process called mechanotransduction. Mechanical force allosterically alters the conformations of mechanosensitive proteins, including integrins, within adhesions to elicit biochemical signals that regulate cellular mechanics and gene expression levels [54,55,56]. These mechanical transmissions are influenced by the resting tension level inside the cell, which is built by the cytoskeletal network [57]. Assuming that TNS2 plays a role in mechanotransduction in podocytes, it is not surprising that the impact of TNS2 deficiency differs depending on the maturity of podocytes, which are highly differentiated cells with an intricate cytoskeletal architecture. ECM remodeling is a major target for mechanoresponsive pathways [54, 58]. In general, mechanical stress facilitates FAK activation [59,60,61,62]. In TNS2-deficient nephropathy, abnormal accumulation of ECM in the GBM is the first abnormality observed in the early postnatal stage, as mentioned above. In addition, FAK activation in glomeruli is one of the molecular characteristics of its pathology [43, 63]. These findings suggest that TNS2 confers mechanical robustness to podocytes; in other words, it elevates the threshold for mechanosensitivity.
The idea that impaired mechanical adjustment of podocytes to biomechanical stress can lead to podocyte alteration is supported by the phenotype of podocyte-specific talin 1-knockout mice [45]. Talin 1 is a mechanosensitive protein that directly links integrins to the actin cytoskeleton [64, 65]. Similar to TNS2, loss of talin 1 in podocytes leads to GBM splitting and thickening, foot process effacement, and mesangial expansion (without mesangial cell proliferation) after birth [45]. Similar pathological features have been commonly reported in mice with podocyte-specific deletion of CD151 [47, 66]. CD151 interacts with laminin-binding integrins (such as integrin α3β1), and involved in the cellular mechanics [67, 68]. In contrast, podocyte-specific deletion of either subunit of integrin α3β1, which is a major type in podocytes [69], leads to prenatal or perinatal foot process effacement, followed by postnatal GBM thickening; however, it does not lead to obvious mesangial expansion [46, 47]. The absence of CD151 reduces the ability of integrin α3β1 to bind to laminin α5β2γ1 [70], a major ligand for integrin α3β1 and a core component of the GBM [71, 72]. Intraglomerular pressure markedly influences the glomerular pathology in CD151-knockout mice [73]. TNS2 deficiency also reduces the adhesion of primary cultured podocytes to laminins [32]. As ECM–integrin binding is bidirectionally involved in mechanotransduction [54, 56, 74], it is reasonable that defects in laminin binding are linked to mechanical stress in podocytes. These data indicate that at least two levels of regulation are involved in integrin-mediated podocyte homeostasis: mechanotransduction (including laminin binding) and differentiation. Laminin β2 chain deficiency itself causes Pierson syndrome, characterized by congenital nephrotic syndrome with DMS, in addition to distinct ocular abnormalities [75, 76]. Mice with a mutation in the laminin β2 chain have been reported to exhibit proteinuria before foot process effacement, likely due to increased GBM permeability [77, 78]. Subsequent foot process effacement is considered to be caused by the exposure of podocytes to high plasma protein concentrations [77].
The GBM is composed of four major components: laminin, collagen IV, heparan sulfate proteoglycan, and nidogen [71, 72]. Laminin α5β2γ1 trimers, a major isoform of GBM laminin, are secreted by both podocytes and endothelial cells [79]. They polymerize to form separate networks at each edge of the GBM [80]. Unlike other collagens, collagen IV is detected only in the basement membrane and consists of six genetically distinct α-chains, designated α1(IV) to α6(IV). The chains assemble into only three types of heterotrimers: α1α1α2(IV), α3α4α5(IV), and α5α5α6(IV) [81]. The GBM contains two distinct collagen IV networks. It mainly contains α3α4α5(IV) and marginally contains α1α1α2(IV) [82]. The collagen α3α4α5(IV) network is solely formed by podocytes [83] and is present at the center of the GBM, whereas the α1α1α2(IV) network is localized on the endothelial side of the GBM [80, 82]. Mutations in genes encoding α3(IV), α4(IV), or α5(IV) result in defects in the assembly of the collagen α3α4α5(IV) network and cause Alport syndrome, characterized by glomerulonephropathy and deafness [84, 85]. In Alport syndrome, the collagen α1α1α2(IV) network expands toward the podocyte side of the GBM [80, 82]. Experimental data suggest at least two levels of regulation are involved in the initiation of Alport syndrome: stimulation of collagen receptors (primarily integrin α1β1) by the compensated expansion of the collagen α1α1α2(IV) network [86,87,88] and biomechanical strain associated with abnormalities in the GBM [89,90,91] (reviewed by Funk, Lin, and Miner [92] and Chew and Lennon [93]). Either form of stimulation can induce a phenotypic change in podocytes, including the expression of genes associated with the ECM and matrix metallopeptidases (MMPs). Ectopic laminin α2 accumulation is observed in the affected GBM in patients and animals with Alport syndrome [94, 95] and is considered to be a key initiator of the pathologies [96]. This deposition of laminin α2 promotes the invasion of mesangial cells into the glomerular capillaries [96] and elevates the expression of MMPs, such as MMP-10 and MMP-12, via FAK activation [95]. Both MMP-10 and MMP-12 play a critical role in the progression of Alport syndrome [97, 98]. In particular, MMP-10 is upregulated in the podocytes of various murine models and patients with nephrotic syndromes, including DN, FSGS, and IgA nephropathy (IgAN), and is considered to be a key mediator of foot process effacement [97]. The upregulation of MMP-10 has also been reported in the podocytes of CD151-knockout mice [99] and Tns2-null mutant mice (our unpublished data). Experimental data suggest that MMP-10 leads to podocyte injury through the proteolytic degradation of the podocyte tight junction protein zonula occludens-1 (ZO-1) [97]. On the other hand, MMP-12 can activate other MMPs (such as MMP-2 and MMP-3) through the proteolytic process [100]. To our knowledge, although there is no direct evidence that MMP-12 can activate MMP-10, based on the similarity between the protein structures of MMP-3 and MMP-10 [101], MMP-12 seems to have the ability to directly activate MMP-10. Ultimately, variations in the pathologies of glomerular injury leading to foot process effacement can be attributed to differences in pathways for upregulating MMP-10.
In this respect, laminin α2 deposition in the glomeruli is a characteristic of Alport syndrome but is usually undetectable in other human CKDs, such as DN, MCNS, FSGS, IgAN, membranoproliferative glomerulonephritis type I, and membranous nephropathy [94]. In addition, FAK activation is not common in glomerulonephropathy [102]. Interestingly, both alterations are also observed in CD151-knockout mice [95, 96] and Tns2-null mutant mice [32, 43]. Furthermore, similar to Alport mice, Tns2-null mutant mice show MMP-12 upregulation in podocytes [103]. These data indicate that the laminin α2/FAK/MMP-12/MMP-10 pathway is common in these nephropathies. Based on the abovementioned hypothesis, this occurs because these nephropathies share a common mechanism wherein biomechanical stress in podocytes induces ectopic laminin α2 accumulation (Fig. 2). Histopathologically, TNS2-deficient nephropathy resembles Alport syndrome, except for mesangial cell proliferation. In Alport syndrome, mesangial expansion is accompanied by mesangial cell proliferation [106]. This dissimilarity can be attributed to collagen α1α1α2(IV)-mediated signaling unique to Alport syndrome.
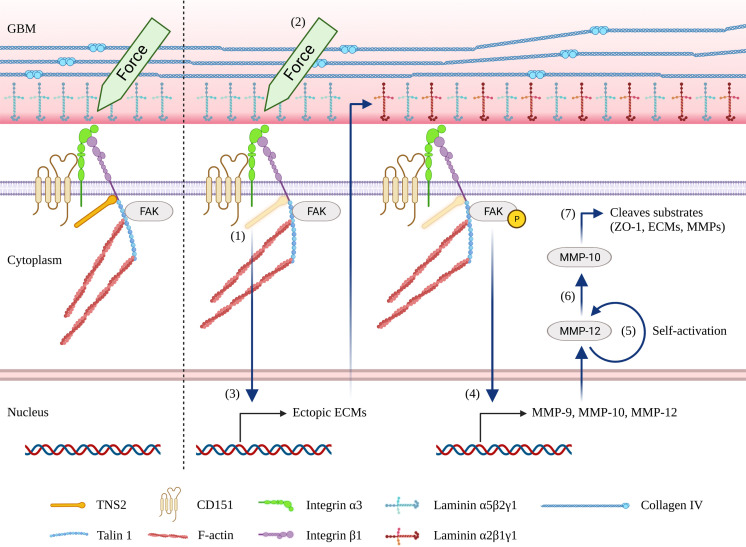
Fig. 2.
A hypothetical model in which mechanical stress leads to foot process effacement in mechanosensitive nephropathy. (1) Loss of integrin-binding focal adhesion proteins, such as tensin 2 (TNS2), CD151, and talin 1, elevates the mechanosensitivity of podocytes. (2) Intraglomerular hydraulic pressure results in biomechanical strain in podocytes. The level of intraglomerular hydraulic pressure is elevated at birth or in the case of hypertension. (3) Highly mechanosensitive podocytes respond to biomechanical strain and trigger abnormal mechanotransduction, resulting in the ectopic expression of the extracellular matrix (ECM) and subsequently causing glomerular basement membrane (GBM) thickening. (4) Accumulated laminin α2 triggers integrin signaling, resulting in an increase in the expression of MMPs, such as matrix metallopeptidase (MMP)-10 and MMP-12, via focal adhesion kinase (FAK) activation. (5) MMP-12 is autolytically processed into an activated form [104, 105]. (6) Active MMP-12 directly activates MMP-10. (7) Active MMP-10 degrades the podocyte tight junction protein zonula occludens-1 (ZO-1), leading to foot process effacement.
Genetic Susceptibility to TNS2-deficient Nephropathy
Tns2-null mutant mice develop glomerular disease in a strain-dependent manner. TNS2 deficiency induces GBM lesions and podocyte foot process effacement and subsequently causes glomerular and tubulointerstitial injuries in the ICGN (the original strain possessing the Tns2nph mutation), FVB/N (FVB), and DBA/2J (D2) strains [27, 63, 107]; however, it causes only modest GBM thickening in the C57BL/6J (B6) and 129+Ter/SvJcl strains [43, 108, 109]. The MSM strain is unaffected by TNS2 deficiency [27]. Similar strain-dependent disparities have been reported in CD151-deficient nephropathy [66, 110, 111]. Interestingly, similar to TNS2 deficiency, CD151 deficiency induces glomerular injuries in an FVB genetic background but not in a B6 genetic background [66]. In Alport syndrome, the rates of progression vary depending on the murine strains. Although Alport mice with a B6 genetic background develop progressive glomerular disease, the progression is slower than that in other strains [112,113,114,115]. These strain-dependent disparities strongly indicate the presence of modifier genes. In addition, a B6 genetic background is almost completely resistant to TNS2- or CD151-deficient nephropathy but is partially resistant to Alport syndrome. Therefore, based on the abovementioned hypothesis, these modifier genes are likely involved in biomechanical stress-mediated laminin α2 deposition in the glomeruli.
Modifier genes
QTL and congenic analyses using resistant B6 and susceptible ICGN mice previously revealed a modifier locus associated with glomerular injury caused by TNS2 deficiency, designated Tpir, on chromosome 2 [116, 117]. Introduction of the B6 allele of Tpir into ICGN mice can alleviate glomerular injury and albuminuria. However, the degree of improvement has been reported to be inferior to that noted in a B6 genetic background itself, wherein no podocyte alteration occurs [116]. This result indicates that Tpir is a minor modifier gene and is not involved in the fundamental step of the pathogenesis. This finding can be attributed to the fact that this genetic analysis focused on the severity of CKD and evaluated the traits related to the late phase of CKD, such as tubulointerstitial injury and renal anemia [117]. The histopathological glomerular injury score optimized for TNS2-deficient nephropathy, characterized by mesangial expansion, is a quantitative trait associated with Tpir. Symptoms of early glomerular injury, such as GBM thickening, can be evaluated using this glomerular injury score; however, in the later phase, the extent of mesangial expansion usually accounts for the largest part in this quantitative assessment. Mesangial expansion eventually disrupts the glomerular structure, and its extent generally correlates with the glomerular filtration rate (GFR) [118]. Therefore, Tpir may modify mesangial expansion. To determine the major modifier genes involved in the fundamental step of the pathogenesis, a genome-wide linkage analysis of Tns2-deficient backcrosses with resistant B6 and susceptible FVB genetic backgrounds was performed in a previous study [119]. In this genetic analysis, N2 backcross mice were phenotyped by assessing the urinary albumin level corresponding to their foot process effacement and not by assessing the amount of urinary albumin excreted, which can be modified by various factors. A marker–trait association test detected significant and suggestive modifier loci designated Tpir2 and Tpir1 were detected on chromosomes 10 and 2, respectively [119]. As the peak marker of Tpir1 is included in Tpir (57.65–78.72 cM), Tpir1 is considered to be identical to Tpir.
Tpir2
Tpir2 is a major modifier locus that may influence the fundamental step of the pathogenesis in TNS2-deficient nephropathy. Notably, the genetic loci associated with urinary albumin excretion in patients with essential hypertension (human 12q23.2 and 19p13.3) [120] reside within the syntenic regions of Tpir2 [119]. From the point of view of cellular mechanics, situations in which podocytes are subjected to biomechanical stress may be common between hypertension and TNS2 deficiency. Hypertension hydraulically loads mechanical strain on podocytes, whereas TNS2 deficiency may make podocytes susceptible to mechanical stress. Amino acid sequence variations between FVB and B6 in the proximity of the peak marker of Tpir2 are shown in Table 1. Here, we focus on the candidate gene Stab2, which resides in proximity to Pah, the peak marker of the human genetic locus for proteinuria in patients with hypertension [120]. Stab2 encodes the primary scavenger receptor for systemic hyaluronan (also known as hyaluronic acid, HA) [122]. STAB2 dysfunction results in a large increase in circulating HA [123, 124]. Although no data are available regarding the metabolism of HA in FVB mice, interestingly, susceptible D2 mice exhibit 10 times higher concentrations of plasma HA than B6 or 129S6 mice because of the ectopic expression of Stab2 triggered by an intracisternal A particle element [125, 126]. Furthermore, TNS2 deficiency can be dominantly inherited in STAB2-knockout mice with an FVB genetic background (our unpublished data). These data suggest that an increased level of systemic HA strongly modifies TNS2-deficient nephropathy; however, whether Stab2 is a causative gene for Tpir2 remains unknown. The biological functions of HA have been studied in nephrological research on inflammation and fibrosis (reviewed by Kaul et al. [127]). However, the direct effects of HA on podocytes remain unknown. On the other hand, pulmonary artery smooth muscle cells have been reported to exhibit changes in terms of cell stiffness, cell proliferation, and cell motility when exposed to HA fragments [128]. Thus, HA has the potential to alter the cellular mechanics of podocytes.
Table 1. Amino acid sequence variations between FVB/N (FVB) and C57BL/6J (B6) in the proximity of the peak marker of Tpir2 (10:76.0–88.7 Mbp).
| Chr | Position (bp) | SNP | B6 | FVB | Gene | Transcript | Change type | Mutation |
|---|---|---|---|---|---|---|---|---|
| 10 | 81643563 | rs255887930 | T | TGCAGAGGCGGAG | Ankrd24 | NM_027480 | Inframe indel | A786_E789dup |
| 10 | 82226062 | rs29382863 | A | T | Zfp938 | NM_001105557 | Non-synonymous | D241E |
| 10 | 82226120 | rs29331622 | C | T | Zfp938 | NM_001105557 | Non-synonymous | C222Y |
| 10 | 82282677 | rs29383954 | T | C | LOC102635990 | XM_006514360 | Non-synonymous | K4833R |
| 10 | 82284600 | rs47061592 | G | C | LOC102635990 | XM_006514360 | Non-synonymous | S4192C |
| 10 | 82284880 | rs47781362 | T | C | LOC102635990 | XM_006514360 | Non-synonymous | I4099V |
| 10 | 82285470 | rs251725877 | TCAG | T | LOC102635990 | XM_006514360 | Inframe indel | T3901_E3902delinsK |
| 10 | 82285510 | rs29340367 | A | G | LOC102635990 | XM_006514360 | Non-synonymous | S3889P |
| 10 | 82287454 | rs29350159 | C | T | LOC102635990 | XM_006514360 | Non-synonymous | A3241T |
| 10 | 82289581 | rs29327471 | T | C | LOC102635990 | XM_006514360 | Non-synonymous | T2532A |
| 10 | 82289593 | rs29349975 | T | C | LOC102635990 | XM_006514360 | Non-synonymous | I2528V |
| 10 | 82290994 | rs224869780 | T | TTGTCAT | LOC102635990 | XM_006514360 | Inframe indel | S2060_F2061insMT |
| 10 | 82291375 | rs29339242 | T | C | LOC102635990 | XM_006514360 | Non-synonymous | M1934V |
| 10 | 82292155 | rs50412724 | G | A | LOC102635990 | XM_006514360 | Non-synonymous | P1674S |
| 10 | 82293640 | rs29348544 | A | T | LOC102635990 | XM_006514360 | Non-synonymous | S1179T |
| 10 | 82294805 | rs29337081 | T | G | LOC102635990 | XM_006514360 | Non-synonymous | R790S |
| 10 | 82295226 | rs29370034 | C | T | LOC102635990 | XM_006514360 | Non-synonymous | R650K |
| 10 | 82295391 | rs29328221 | C | T | LOC102635990 | XM_006514360 | Non-synonymous | R595K |
| 10 | 82492194 | rs47627276 | G | T | Gm1553 | NM_001255990 | Non-synonymous | L60M |
| 10 | 82638847 | rs49510163 | A | C | Tdg | NM_011561 | Non-synonymous | E33A |
| 10 | 82638847 | rs49510163 | A | C | Tdg | NM_172552 | Non-synonymous | E57A |
| 10 | 82647345 | rs47249452 | G | A | Tdg | NM_011561 | Non-synonymous | A293T |
| 10 | 82647347 | rs49056941 | G | C | Tdg | NM_011561 | Non-synonymous | |
| 10 | 82647345 | rs47249452 | G | A | Tdg | NM_172552 | Non-synonymous | A317T |
| 10 | 82647347 | rs49056941 | G | C | Tdg | NM_172552 | Non-synonymous | |
| 10 | 82648610 | rs47946519 | G | A | Tdg | NM_011561 | Non-synonymous | A362T |
| 10 | 82648610 | rs47946519 | G | A | Tdg | NM_172552 | Non-synonymous | A386T |
| 10 | 82648616 | rs47182293 | A | G | Tdg | NM_011561 | Non-synonymous | S364G |
| 10 | 82648616 | rs47182293 | A | G | Tdg | NM_172552 | Non-synonymous | S388G |
| 10 | 82670307 | rs29334621 | T | C | Glt8d2 | NM_029102 | Non-synonymous | H30R |
| 10 | 83508124 | rs13480674 | A | G | Aldh1l2 | NM_153543 | Non-synonymous | Y476H |
| 10 | 85388329 | rs29382636 | T | C | Btbd11 | NM_028709 | Non-synonymous | V334A |
| 10 | 86294645 | rs264253067 | AA | - | Syn3 | NM_001164495 | Frameshift | F238Yfs*14 |
| 10 | 86467097 | rs30199006 | C | T | Syn3 | NM_001164495 | Non-synonymous | G65S |
| 10 | 86467097 | rs30199006 | C | T | Syn3 | NM_013722 | Non-synonymous | G65S |
| 10 | 86539954 | rs265230719 | C | A | Gm6729 | NM_001384224 | Non-synonymous | A461S |
| 10 | 86539981 | rs387036780 | G | C | Gm6729 | NM_001384224 | Non-synonymous | L452V |
| 10 | 86541308 | rs259617967 | T | G | Gm6729 | NM_001384224 | Non-synonymous | R9S |
| 10 | 86541328 | rs216762171 | T | G | Gm6729 | NM_001384224 | Non-synonymous | S3R |
| 10 | 86655945 | rs220212569 | A | T | Gm5174 | NM_001384216 | Non-synonymous | S3C |
| 10 | 86656626 | rs30217026 | T | A | Gm5174 | NM_001384216 | Non-synonymous | Y230N |
| 10 | 86656805 | rs29377958 | G | T | Gm5174 | NM_001384216 | Non-synonymous | E289D |
| 10 | 86656930 | rs386949678 | T | C | Gm5174 | NM_001384216 | Non-synonymous | V331A |
| 10 | 86657064 | rs387395855 | C | T | Gm5174 | NM_001384216 | Non-synonymous | L376F |
| 10 | 86691704 | rs250907279 | G | C | Hsp90b1 | NM_011631 | Non-synonymous | D769E |
| 10 | 86731086 | rs30231257 | G | A | Ttc41 | NM_001003910, NM_153595 | Non-synonymous | A539T |
| 10 | 86776504 | rs6412755 | G | C | Ttc41 | NM_001003910 | Non-synonymous | A1214P |
| 10 | 86776652 | rs6152391 | T | C | Ttc41 | NM_001003910 | Non-synonymous | L1263P |
| 10 | 86776664 | rs6152415 | T | C | Ttc41 | NM_001003910 | Non-synonymous | V1267A |
| 10 | 86779206 | rs231797092 | T | G | Nt5dc3 | NM_175331 | Non-synonymous | S33A |
| 10 | 86779267 | rs216734988 | G | A | Nt5dc3 | NM_175331 | Non-synonymous | S53N |
| 10 | 86848120 | rs253335427 | C | T | Stab2 | NM_138673 | Non-synonymous | V2392I |
| 10 | 86850628 | rs252154668 | C | T | Stab2 | NM_138673 | Non-synonymous | D2313N |
| 10 | 86850851 | rs249686084 | A | C | Stab2 | NM_138673 | Non-synonymous | I2276M |
| 10 | 86858183 | rs30231575 | T | C | Stab2 | NM_138673 | Non-synonymous | M2065V |
| 10 | 86865017 | rs30230495 | C | T | Stab2 | NM_138673 | Non-synonymous | R1917K |
| 10 | 86872653 | rs30236399 | C | T | Stab2 | NM_138673 | Non-synonymous | V1683M |
| 10 | 86872728 | rs30236402 | T | C | Stab2 | NM_138673 | Non-synonymous | K1658E |
| 10 | 86873894 | rs30238216 | T | C | Stab2 | NM_138673 | Non-synonymous | Q1629R |
| 10 | 86897981 | rs211832720 | T | C | Stab2 | NM_138673 | Non-synonymous | I1326V |
| 10 | 86905631 | rs29367139 | T | C | Stab2 | NM_138673 | Non-synonymous | Q1208R |
| 10 | 86933327 | rs30245789 | C | T | Stab2 | NM_138673 | Non-synonymous | S921N |
| 10 | 86938052 | rs30245017 | A | G | Stab2 | NM_138673 | Non-synonymous | I805T |
| 10 | 86969772 | rs30243498 | T | C | Stab2 | NM_138673 | Non-synonymous | K237E |
| 10 | 86979990 | rs30242264 | C | T | Stab2 | NM_138673 | Non-synonymous | R151H |
| 10 | 87165105 | rs30247300 | C | T | 1700113H08Rik | NM_029685 | Non-synonymous | T53M |
| 10 | 87225901 | rs30252317 | T | A | 1700113H08Rik | NM_029685 | Non-synonymous | M71K |
| 10 | 87226012 | rs214268401 | C | T | 1700113H08Rik | NM_029685 | Non-synonymous | S108L |
| 10 | 88091308 | rs49617371 | G | A | Pmch | NM_029971 | Non-synonymous | V58I |
| 10 | 88093169 | rs48863032 | C | T | Parpbp | NM_029249 | Non-synonymous | V470I |
| 10 | 88133128 | rs29314114 | T | G | Parpbp | NM_029249 | Non-synonymous | E152A |
| 10 | 88245799 | rs387426886 | G | A | Washc3 | NM_026070 | Non-synonymous | V182I |
| 10 | 88245799 | rs387426886 | G | A | Washc3 | NM_001122960 | Non-synonymous | V181I |
Single nucleotide polymorphism (SNP) and indel data are from the Mouse Phenome Database (RRIDSCR_003212) [121]. Position data are based on Mus musculus genome assembly GRCm38 (mm10). Chr, chromosome.
Conclusion
TNS2-deficient nephropathy resembles podocyte-specific CD151- or talin 1-deficient nephropathy in that its pathology is characterized by early postnatal GBM thickening, foot process effacement, and mesangial expansion. These three integrin-binding focal adhesion proteins do not seem to be involved in podocyte differentiation; however, they seem to be involved in podocyte mechanics. A deficiency of either molecule may elevate the mechanosensitivity of podocytes, which can give rise to abnormal mechanotransduction in response to normal biomechanical strain. A similar mechanism seems to partially underlie Alport syndrome. Indeed, these nephropathies share common molecular pathologies, such as laminin α2 deposition and FAK activation in the glomeruli, which are not commonly observed in other glomerulonephropathies. Finally, we postulate that TNS2 deficiency promotes mechanical stress-induced activation of the laminin α2/FAK/MMP-12/MMP-10 axis and that MMP-10 activation eventually leads to podocyte foot process effacement. The strict strain-dependent onset of TNS2-deficient nephropathy may be attributed to modifier genes that can alter podocyte mechanics.
Acknowledgments
We wish to thank Dr. Shigeru Kakuta for his recommendation for this award. We thank Drs. Makoto Sugiyama, Nakano Kenta, and Tadashi Okamura for their helpful discussions and thank our lab members for their technical assistance. This work was supported by the Ministry of Education, Culture, Sports, Science and Technology (MEXT) and JSPS KAKENHI Grant Numbers 16K16606 (to HS), 20K08642 (to HS), 25430083 (to NS), and 20K06474 (to NS).
References
- 1.Liao YC, Lo SH. Tensins - emerging insights into their domain functions, biological roles and disease relevance. J Cell Sci. 2021; 134: jcs254029. doi: 10.1242/jcs.254029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haynie DT. Molecular physiology of the tensin brotherhood of integrin adaptor proteins. Proteins. 2014; 82: 1113–1127. doi: 10.1002/prot.24560 [DOI] [PubMed] [Google Scholar]
- 3.Critchley DR. Focal adhesions - the cytoskeletal connection. Curr Opin Cell Biol. 2000; 12: 133–139. doi: 10.1016/S0955-0674(99)00067-8 [DOI] [PubMed] [Google Scholar]
- 4.Zaidel-Bar R, Itzkovitz S, Ma’ayan A, Iyengar R, Geiger B. Functional atlas of the integrin adhesome. Nat Cell Biol. 2007; 9: 858–867. doi: 10.1038/ncb0807-858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wehrle-Haller B. Structure and function of focal adhesions. Curr Opin Cell Biol. 2012; 24: 116–124. doi: 10.1016/j.ceb.2011.11.001 [DOI] [PubMed] [Google Scholar]
- 6.Papusheva E, Mello de Queiroz F, Dalous J, Han Y, Esposito A, Jares-Erijmanxa EA, et al. Dynamic conformational changes in the FERM domain of FAK are involved in focal-adhesion behavior during cell spreading and motility. J Cell Sci. 2009; 122: 656–666. doi: 10.1242/jcs.028738 [DOI] [PubMed] [Google Scholar]
- 7.Legate KR, Fässler R. Mechanisms that regulate adaptor binding to β-integrin cytoplasmic tails. J Cell Sci. 2009; 122: 187–198. doi: 10.1242/jcs.041624 [DOI] [PubMed] [Google Scholar]
- 8.Harburger DS, Calderwood DA. Integrin signalling at a glance. J Cell Sci. 2009; 122: 159–163. doi: 10.1242/jcs.018093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Howe A, Aplin AE, Alahari SK, Juliano RL. Integrin signaling and cell growth control. Curr Opin Cell Biol. 1998; 10: 220–231. doi: 10.1016/S0955-0674(98)80144-0 [DOI] [PubMed] [Google Scholar]
- 10.Park MJ, Sheng R, Silkov A, Jung DJ, Wang ZG, Xin Y, et al. SH2 Domains Serve as Lipid-Binding Modules for pTyr-Signaling Proteins. Mol Cell. 2016; 62: 7–20. doi: 10.1016/j.molcel.2016.01.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim E, Kim DH, Singaram I, Jeong H, Koh A, Lee J, et al. Cellular phosphatase activity of C1-Ten/Tensin2 is controlled by Phosphatidylinositol-3,4,5-triphosphate binding through the C1-Ten/Tensin2 SH2 domain. Cell Signal. 2018; 51: 130–138. doi: 10.1016/j.cellsig.2018.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leone M, Yu EC, Liddington RC, Pasquale EB, Pellecchia M. The PTB domain of tensin: NMR solution structure and phosphoinositides binding studies. Biopolymers. 2008; 89: 86–92. doi: 10.1002/bip.20862 [DOI] [PubMed] [Google Scholar]
- 13.Calderwood DA, Fujioka Y, de Pereda JM, García-Alvarez B, Nakamoto T, Margolis B, et al. Integrin beta cytoplasmic domain interactions with phosphotyrosine-binding domains: a structural prototype for diversity in integrin signaling. Proc Natl Acad Sci USA. 2003; 100: 2272–2277. doi: 10.1073/pnas.262791999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Katz M, Amit I, Citri A, Shay T, Carvalho S, Lavi S, et al. A reciprocal tensin-3-cten switch mediates EGF-driven mammary cell migration. Nat Cell Biol. 2007; 9: 961–969. doi: 10.1038/ncb1622 [DOI] [PubMed] [Google Scholar]
- 15.Seo EY, Jin SP, Kim YK, Lee H, Han S, Lee DH, et al. Integrin-β4-TNS4-Focal Adhesion Kinase Signaling Mediates Keratinocyte Proliferation in Human Skin. J Invest Dermatol. 2017; 137: 763–766. doi: 10.1016/j.jid.2016.10.039 [DOI] [PubMed] [Google Scholar]
- 16.McCleverty CJ, Lin DC, Liddington RC. Structure of the PTB domain of tensin1 and a model for its recruitment to fibrillar adhesions. Protein Sci. 2007; 16: 1223–1229. doi: 10.1110/ps.072798707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hall EH, Balsbaugh JL, Rose KL, Shabanowitz J, Hunt DF, Brautigan DL. Comprehensive analysis of phosphorylation sites in Tensin1 reveals regulation by p38MAPK. Mol Cell Proteomics. 2010; 9: 2853–2863. doi: 10.1074/mcp.M110.003665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qian X, Li G, Vass WC, Papageorge A, Walker RC, Asnaghi L, et al. The Tensin-3 protein, including its SH2 domain, is phosphorylated by Src and contributes to tumorigenesis and metastasis. Cancer Cell. 2009; 16: 246–258. doi: 10.1016/j.ccr.2009.07.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qian X, Li G, Asmussen HK, Asnaghi L, Vass WC, Braverman R, et al. Oncogenic inhibition by a deleted in liver cancer gene requires cooperation between tensin binding and Rho-specific GTPase-activating protein activities. Proc Natl Acad Sci USA. 2007; 104: 9012–9017. doi: 10.1073/pnas.0703033104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liao YC, Si L, deVere White RW, Lo SH. The phosphotyrosine-independent interaction of DLC-1 and the SH2 domain of cten regulates focal adhesion localization and growth suppression activity of DLC-1. J Cell Biol. 2007; 176: 43–49. doi: 10.1083/jcb.200608015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chan LK, Ko FCF, Ng IOL, Yam JWP. Deleted in liver cancer 1 (DLC1) utilizes a novel binding site for Tensin2 PTB domain interaction and is required for tumor-suppressive function. PLoS One. 2009; 4: e5572. doi: 10.1371/journal.pone.0005572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clark K, Howe JD, Pullar CE, Green JA, Artym VV, Yamada KM, et al. Tensin 2 modulates cell contractility in 3D collagen gels through the RhoGAP DLC1. J Cell Biochem. 2010; 109: 808–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Winograd-Katz SE, Fässler R, Geiger B, Legate KR. The integrin adhesome: from genes and proteins to human disease. Nat Rev Mol Cell Biol. 2014; 15: 273–288. doi: 10.1038/nrm3769 [DOI] [PubMed] [Google Scholar]
- 24.Best MG, Sol N, Kooi I, Tannous J, Westerman BA, Rustenburg F, et al. RNA-Seq of Tumor-Educated Platelets Enables Blood-Based Pan-Cancer, Multiclass, and Molecular Pathway Cancer Diagnostics. Cancer Cell. 2015; 28: 666–676. doi: 10.1016/j.ccell.2015.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hiura K, Sakanoue A, Kontani S, Takahashi Y, Watanabe M, Nakano K, et al. Suppression of tensin 2 promotes intestinal tumorigenesis by liberating integrin-linked kinase-induced nuclear translocation of β-catenin. Transl Regul Sci. 2020; 2: 51–59. [Google Scholar]
- 26.Ashraf S, Kudo H, Rao J, Kikuchi A, Widmeier E, Lawson JA, et al. Mutations in six nephrosis genes delineate a pathogenic pathway amenable to treatment. Nat Commun. 2018; 9: 1960. doi: 10.1038/s41467-018-04193-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cho AR, Uchio-Yamada K, Torigai T, Miyamoto T, Miyoshi I, Matsuda J, et al. Deficiency of the tensin2 gene in the ICGN mouse: an animal model for congenital nephrotic syndrome. Mamm Genome. 2006; 17: 407–416. doi: 10.1007/s00335-005-0167-z [DOI] [PubMed] [Google Scholar]
- 28.Marusugi K, Nakano K, Sasaki H, Kimura J, Yanobu-Takanashi R, Okamura T, et al. Functional validation of tensin2 SH2-PTB domain by CRISPR/Cas9-mediated genome editing. J Vet Med Sci. 2016; 78: 1413–1420. doi: 10.1292/jvms.16-0205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lattin JE, Schroder K, Su AI, Walker JR, Zhang J, Wiltshire T, et al. Expression analysis of G Protein-Coupled Receptors in mouse macrophages. Immunome Res. 2008; 4: 5. doi: 10.1186/1745-7580-4-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Uhlén M, Fagerberg L, Hallström BM, Lindskog C, Oksvold P, Mardinoglu A, et al. Proteomics. Tissue-based map of the human proteome. Science. 2015; 347: 1260419. doi: 10.1126/science.1260419 [DOI] [PubMed] [Google Scholar]
- 31.Pavenstädt H, Kriz W, Kretzler M. Cell biology of the glomerular podocyte. Physiol Rev. 2003; 83: 253–307. doi: 10.1152/physrev.00020.2002 [DOI] [PubMed] [Google Scholar]
- 32.Uchio-Yamada K, Yasuda K, Monobe Y, Akagi KI, Suzuki O, Manabe N. Tensin2 is important for podocyte-glomerular basement membrane interaction and integrity of the glomerular filtration barrier. Am J Physiol Renal Physiol. 2020; 318: F1520–F1530. doi: 10.1152/ajprenal.00055.2020 [DOI] [PubMed] [Google Scholar]
- 33.Nishino T, Sasaki N, Chihara M, Nagasaki K, Torigoe D, Kon Y, et al. Distinct distribution of the tensin family in the mouse kidney and small intestine. Exp Anim. 2012; 61: 525–532. doi: 10.1538/expanim.61.525 [DOI] [PubMed] [Google Scholar]
- 34.Ogura A, Fujimura H, Asano T, Koura M, Naito I, Kobayashi Y. Early ultrastructural glomerular alterations in neonatal nephrotic mice (ICGN strain). Vet Pathol. 1995; 32: 321–323. doi: 10.1177/030098589503200317 [DOI] [PubMed] [Google Scholar]
- 35.Ogura A, Asano T, Matsuda J, Koura M, Nakagawa M, Kawaguchi H, et al. An electron microscopic study of glomerular lesions in hereditary nephrotic mice (ICGN strain). Virchows Arch A Pathol Anat Histopathol. 1990; 417: 223–228. doi: 10.1007/BF01600137 [DOI] [PubMed] [Google Scholar]
- 36.Ogura A, Asano T, Matsuda J, Fujimura H. Evolution of glomerular lesions in nephrotic ICGN mice: serial biopsy study with electron microscopy. J Vet Med Sci. 1991; 53: 513–515. doi: 10.1292/jvms.53.513 [DOI] [PubMed] [Google Scholar]
- 37.Mizuno S, Mizuno-Horikawa Y, Yue BF, Okamoto M, Kurosawa T. Nephrotic mice (ICGN strain): a model of diffuse mesangial sclerosis in infantile nephrotic syndrome. Am J Nephrol. 1999; 19: 73–82. doi: 10.1159/000013430 [DOI] [PubMed] [Google Scholar]
- 38.Kato T, Mizuno S, Taketo MM, Kurosawa TM. The possible involvement of tensin2 in the expression and extension of nephrin by glomerular podocytes in mice. Biomed Res. 2008; 29: 279–287. doi: 10.2220/biomedres.29.279 [DOI] [PubMed] [Google Scholar]
- 39.Ogura A, Asano T, Matsuda J, Takano K, Nakagawa M, Fukui M. Characteristics of mutant mice (ICGN) with spontaneous renal lesions: a new model for human nephrotic syndrome. Lab Anim. 1989; 23: 169–174. doi: 10.1258/002367789780863628 [DOI] [PubMed] [Google Scholar]
- 40.Uchio K, Manabe N, Tamura K, Miyamoto M, Yamaguchi M, Ogura A, et al. Decreased matrix metalloproteinase activity in the kidneys of hereditary nephrotic mice (ICGN strain). Nephron. 2000; 86: 145–151. doi: 10.1159/000045733 [DOI] [PubMed] [Google Scholar]
- 41.Goto Y, Manabe N, Uchio-Yamada K, Yamaguchi-Yamada M, Inoue N, Yamamoto Y, et al. Augmented cytoplasmic Smad4 induces acceleration of TGF-beta1 signaling in renal tubulointerstitial cells of hereditary nephrotic ICGN mice with chronic renal fibrosis; possible role for myofibroblastic differentiation. Cell Tissue Res. 2004; 315: 209–221. doi: 10.1007/s00441-003-0824-z [DOI] [PubMed] [Google Scholar]
- 42.Mizuno S, Matsumoto K, Kurosawa T, Mizuno-Horikawa Y, Nakamura T. Reciprocal balance of hepatocyte growth factor and transforming growth factor-β 1 in renal fibrosis in mice. Kidney Int. 2000; 57: 937–948. doi: 10.1038/sj.ki.4491416 [DOI] [PubMed] [Google Scholar]
- 43.Uchio-Yamada K, Sawada K, Tamura K, Katayama S, Monobe Y, Yamamoto Y, et al. Tenc1-deficient mice develop glomerular disease in a strain-specific manner. Nephron, Exp Nephrol. 2013; 123: 22–33. doi: 10.1159/000354058 [DOI] [PubMed] [Google Scholar]
- 44.Sasaki H, Takahashi Y, Ogawa T, Hiura K, Nakano K, Sugiyama M, et al. Deletion of the Tensin2 SH2-PTB domain, but not the loss of its PTPase activity, induces podocyte injury in FVB/N mouse strain. Exp Anim. 2020; 69: 135–143. doi: 10.1538/expanim.19-0101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tian X, Kim JJ, Monkley SM, Gotoh N, Nandez R, Soda K, et al. Podocyte-associated talin1 is critical for glomerular filtration barrier maintenance. J Clin Invest. 2014; 124: 1098–1113. doi: 10.1172/JCI69778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pozzi A, Jarad G, Moeckel GW, Coffa S, Zhang X, Gewin L, et al. Beta1 integrin expression by podocytes is required to maintain glomerular structural integrity. Dev Biol. 2008; 316: 288–301. doi: 10.1016/j.ydbio.2008.01.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sachs N, Kreft M, van den Bergh Weerman MA, Beynon AJ, Peters TA, Weening JJ, et al. Kidney failure in mice lacking the tetraspanin CD151. J Cell Biol. 2006; 175: 33–39. doi: 10.1083/jcb.200603073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dai C, Stolz DB, Bastacky SI, St-Arnaud R, Wu C, Dedhar S, et al. Essential role of integrin-linked kinase in podocyte biology: Bridging the integrin and slit diaphragm signaling. J Am Soc Nephrol. 2006; 17: 2164–2175. doi: 10.1681/ASN.2006010033 [DOI] [PubMed] [Google Scholar]
- 49.El-Aouni C, Herbach N, Blattner SM, Henger A, Rastaldi MP, Jarad G, et al. Podocyte-specific deletion of integrin-linked kinase results in severe glomerular basement membrane alterations and progressive glomerulosclerosis. J Am Soc Nephrol. 2006; 17: 1334–1344. doi: 10.1681/ASN.2005090921 [DOI] [PubMed] [Google Scholar]
- 50.Lee J, Koh A, Jeong H, Kim E, Ha TS, Saleem MA, et al. C1-Ten is a PTPase of nephrin, regulating podocyte hypertrophy through mTORC1 activation. Sci Rep. 2017; 7: 12346. doi: 10.1038/s41598-017-12382-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gödel M, Hartleben B, Herbach N, Liu S, Zschiedrich S, Lu S, et al. Role of mTOR in podocyte function and diabetic nephropathy in humans and mice. J Clin Invest. 2011; 121: 2197–2209. doi: 10.1172/JCI44774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Inoki K, Mori H, Wang J, Suzuki T, Hong S, Yoshida S, et al. mTORC1 activation in podocytes is a critical step in the development of diabetic nephropathy in mice. J Clin Invest. 2011; 121: 2181–2196. doi: 10.1172/JCI44771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tóth-Heyn P, Drukker A, Guignard JP. The stressed neonatal kidney: from pathophysiology to clinical management of neonatal vasomotor nephropathy. Pediatr Nephrol. 2000; 14: 227–239. doi: 10.1007/s004670050048 [DOI] [PubMed] [Google Scholar]
- 54.Schwartz MA. Integrins and extracellular matrix in mechanotransduction. Cold Spring Harb Perspect Biol. 2010; 2: a005066. doi: 10.1101/cshperspect.a005066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sun Z, Guo SS, Fässler R. Integrin-mediated mechanotransduction. J Cell Biol. 2016; 215: 445–456. doi: 10.1083/jcb.201609037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Seetharaman S, Etienne-Manneville S. Integrin diversity brings specificity in mechanotransduction. Biol Cell. 2018; 110: 49–64. doi: 10.1111/boc.201700060 [DOI] [PubMed] [Google Scholar]
- 57.Ingber DE. Cellular mechanotransduction: putting all the pieces together again. FASEB J. 2006; 20: 811–827. doi: 10.1096/fj.05-5424rev [DOI] [PubMed] [Google Scholar]
- 58.Chiquet M, Renedo AS, Huber F, Flück M. How do fibroblasts translate mechanical signals into changes in extracellular matrix production? Matrix Biol. 2003; 22: 73–80. doi: 10.1016/S0945-053X(03)00004-0 [DOI] [PubMed] [Google Scholar]
- 59.Zhou J, Aponte-Santamaría C, Sturm S, Bullerjahn JT, Bronowska A, Gräter F. Mechanism of Focal Adhesion Kinase Mechanosensing. PLOS Comput Biol. 2015; 11: e1004593. doi: 10.1371/journal.pcbi.1004593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Guilluy C, Swaminathan V, Garcia-Mata R, O’Brien ET, Superfine R, Burridge K. The Rho GEFs LARG and GEF-H1 regulate the mechanical response to force on integrins. Nat Cell Biol. 2011; 13: 722–727. doi: 10.1038/ncb2254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, et al. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005; 8: 241–254. doi: 10.1016/j.ccr.2005.08.010 [DOI] [PubMed] [Google Scholar]
- 62.Liang W, Ren K, Liu F, Cui W, Wang Q, Chen Z, et al. Periodic mechanical stress stimulates the FAK mitogenic signal in rat chondrocytes through ERK1/2 activity. Cell Physiol Biochem. 2013; 32: 915–930. doi: 10.1159/000354495 [DOI] [PubMed] [Google Scholar]
- 63.Uchio-Yamada K, Monobe Y, Akagi K, Yamamoto Y, Ogura A, Manabe N. Tensin2-deficient mice on FVB/N background develop severe glomerular disease. J Vet Med Sci. 2016; 78: 811–818. doi: 10.1292/jvms.15-0442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Elosegui-Artola A, Oria R, Chen Y, Kosmalska A, Pérez-González C, Castro N, et al. Mechanical regulation of a molecular clutch defines force transmission and transduction in response to matrix rigidity. Nat Cell Biol. 2016; 18: 540–548. doi: 10.1038/ncb3336 [DOI] [PubMed] [Google Scholar]
- 65.del Rio A, Perez-Jimenez R, Liu R, Roca-Cusachs P, Fernandez JM, Sheetz MP. Stretching single talin rod molecules activates vinculin binding. Science. 2009; 323: 638–641. doi: 10.1126/science.1162912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Baleato RM, Guthrie PL, Gubler MC, Ashman LK, Roselli S. Deletion of CD151 results in a strain-dependent glomerular disease due to severe alterations of the glomerular basement membrane. Am J Pathol. 2008; 173: 927–937. doi: 10.2353/ajpath.2008.071149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kazarov AR, Yang X, Stipp CS, Sehgal B, Hemler ME. An extracellular site on tetraspanin CD151 determines α 3 and α 6 integrin-dependent cellular morphology. J Cell Biol. 2002; 158: 1299–1309. doi: 10.1083/jcb.200204056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang F, Michaelson JE, Moshiach S, Sachs N, Zhao W, Sun Y, et al. Tetraspanin CD151 maintains vascular stability by balancing the forces of cell adhesion and cytoskeletal tension. Blood. 2011; 118: 4274–4284. doi: 10.1182/blood-2011-03-339531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Adler S. Characterization of glomerular epithelial cell matrix receptors. Am J Pathol. 1992; 141: 571–578. [PMC free article] [PubMed] [Google Scholar]
- 70.Nishiuchi R, Sanzen N, Nada S, Sumida Y, Wada Y, Okada M, et al. Potentiation of the ligand-binding activity of integrin alpha3beta1 via association with tetraspanin CD151. Proc Natl Acad Sci USA. 2005; 102: 1939–1944. doi: 10.1073/pnas.0409493102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Miner JH. The glomerular basement membrane. Exp Cell Res. 2012; 318: 973–978. doi: 10.1016/j.yexcr.2012.02.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Naylor RW, Morais MRPT, Lennon R. Complexities of the glomerular basement membrane. Nat Rev Nephrol. 2021; 17: 112–127. doi: 10.1038/s41581-020-0329-y [DOI] [PubMed] [Google Scholar]
- 73.Sachs N, Claessen N, Aten J, Kreft M, Teske GJD, Koeman A, et al. Blood pressure influences end-stage renal disease of Cd151 knockout mice. J Clin Invest. 2012; 122: 348–358. doi: 10.1172/JCI58878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Luo BH, Springer TA. Integrin structures and conformational signaling. Curr Opin Cell Biol. 2006; 18: 579–586. doi: 10.1016/j.ceb.2006.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zenker M, Aigner T, Wendler O, Tralau T, Müntefering H, Fenski R, et al. Human laminin β2 deficiency causes congenital nephrosis with mesangial sclerosis and distinct eye abnormalities. Hum Mol Genet. 2004; 13: 2625–2632. doi: 10.1093/hmg/ddh284 [DOI] [PubMed] [Google Scholar]
- 76.Zenker M, Tralau T, Lennert T, Pitz S, Mark K, Madlon H, et al. Congenital nephrosis, mesangial sclerosis, and distinct eye abnormalities with microcoria: an autosomal recessive syndrome. Am J Med Genet A. 2004; 130A: 138–145. doi: 10.1002/ajmg.a.30310 [DOI] [PubMed] [Google Scholar]
- 77.Jarad G, Cunningham J, Shaw AS, Miner JH. Proteinuria precedes podocyte abnormalities inLamb2-/- mice, implicating the glomerular basement membrane as an albumin barrier. J Clin Invest. 2006; 116: 2272–2279. doi: 10.1172/JCI28414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Funk SD, Bayer RH, McKee KK, Okada K, Nishimune H, Yurchenco PD, et al. A deletion in the N-terminal polymerizing domain of laminin β2 is a new mouse model of chronic nephrotic syndrome. Kidney Int. 2020; 98: 133–146. doi: 10.1016/j.kint.2020.01.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.St John PL, Abrahamson DR. Glomerular endothelial cells and podocytes jointly synthesize laminin-1 and -11 chains. Kidney Int. 2001; 60: 1037–1046. doi: 10.1046/j.1523-1755.2001.0600031037.x [DOI] [PubMed] [Google Scholar]
- 80.Suleiman H, Zhang L, Roth R, Heuser JE, Miner JH, Shaw AS, et al. Nanoscale protein architecture of the kidney glomerular basement membrane. eLife. 2013; 2: e01149. doi: 10.7554/eLife.01149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Khoshnoodi J, Pedchenko V, Hudson BG. Mammalian collagen IV. Microsc Res Tech. 2008; 71: 357–370. doi: 10.1002/jemt.20564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kashtan CE, Kim Y. Distribution of the α 1 and α 2 chains of collagen IV and of collagens V and VI in Alport syndrome. Kidney Int. 1992; 42: 115–126. doi: 10.1038/ki.1992.269 [DOI] [PubMed] [Google Scholar]
- 83.Abrahamson DR, Hudson BG, Stroganova L, Borza DB, St John PL. Cellular origins of type IV collagen networks in developing glomeruli. J Am Soc Nephrol. 2009; 20: 1471–1479. doi: 10.1681/ASN.2008101086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kashtan CE. Alport syndrome. An inherited disorder of renal, ocular, and cochlear basement membranes. Medicine (Baltimore). 1999; 78: 338–360. doi: 10.1097/00005792-199909000-00005 [DOI] [PubMed] [Google Scholar]
- 85.Mochizuki T, Lemmink HH, Mariyama M, Antignac C, Gubler MC, Pirson Y, et al. Identification of mutations in the α 3(IV) and α 4(IV) collagen genes in autosomal recessive Alport syndrome. Nat Genet. 1994; 8: 77–81. doi: 10.1038/ng0994-77 [DOI] [PubMed] [Google Scholar]
- 86.Gross O, Girgert R, Beirowski B, Kretzler M, Kang HG, Kruegel J, et al. Loss of collagen-receptor DDR1 delays renal fibrosis in hereditary type IV collagen disease. Matrix Biol. 2010; 29: 346–356. doi: 10.1016/j.matbio.2010.03.002 [DOI] [PubMed] [Google Scholar]
- 87.Rubel D, Frese J, Martin M, Leibnitz A, Girgert R, Miosge N, et al. Collagen receptors integrin alpha2beta1 and discoidin domain receptor 1 regulate maturation of the glomerular basement membrane and loss of integrin alpha2beta1 delays kidney fibrosis in COL4A3 knockout mice. Matrix Biol. 2014; 34: 13–21. doi: 10.1016/j.matbio.2014.01.006 [DOI] [PubMed] [Google Scholar]
- 88.Cosgrove D, Rodgers K, Meehan D, Miller C, Bovard K, Gilroy A, et al. Integrin α1β1 and transforming growth factor-β1 play distinct roles in alport glomerular pathogenesis and serve as dual targets for metabolic therapy. Am J Pathol. 2000; 157: 1649–1659. doi: 10.1016/S0002-9440(10)64802-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Meehan DT, Delimont D, Cheung L, Zallocchi M, Sansom SC, Holzclaw JD, et al. Biomechanical strain causes maladaptive gene regulation, contributing to Alport glomerular disease. Kidney Int. 2009; 76: 968–976. doi: 10.1038/ki.2009.324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gross O, Beirowski B, Koepke ML, Kuck J, Reiner M, Addicks K, et al. Preemptive ramipril therapy delays renal failure and reduces renal fibrosis in COL4A3-knockout mice with Alport syndrome. Kidney Int. 2003; 63: 438–446. doi: 10.1046/j.1523-1755.2003.00779.x [DOI] [PubMed] [Google Scholar]
- 91.Gross O, Licht C, Anders HJ, Hoppe B, Beck B, Tönshoff B, et al. Study Group Members of the Gesellschaft für Pädiatrische Nephrologie. Early angiotensin-converting enzyme inhibition in Alport syndrome delays renal failure and improves life expectancy. Kidney Int. 2012; 81: 494–501. doi: 10.1038/ki.2011.407 [DOI] [PubMed] [Google Scholar]
- 92.Funk SD, Lin MH, Miner JH. Alport syndrome and Pierson syndrome: Diseases of the glomerular basement membrane. Matrix Biol. 2018; 71–72: 250–261. doi: 10.1016/j.matbio.2018.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chew C, Lennon R. Basement Membrane Defects in Genetic Kidney Diseases. Front Pediatr. 2018; 6: 11. doi: 10.3389/fped.2018.00011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kashtan CE, Kim Y, Lees GE, Thorner PS, Virtanen I, Miner JH. Abnormal glomerular basement membrane laminins in murine, canine, and human Alport syndrome: aberrant laminin alpha2 deposition is species independent. J Am Soc Nephrol. 2001; 12: 252–260. doi: 10.1681/ASN.V122252 [DOI] [PubMed] [Google Scholar]
- 95.Zallocchi M, Johnson BM, Meehan DT, Delimont D, Cosgrove D. α1β1 integrin/Rac1-dependent mesangial invasion of glomerular capillaries in Alport syndrome. Am J Pathol. 2013; 183: 1269–1280. doi: 10.1016/j.ajpath.2013.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Delimont D, Dufek BM, Meehan DT, Zallocchi M, Gratton MA, Phillips G, et al. Laminin α2-mediated focal adhesion kinase activation triggers Alport glomerular pathogenesis. PLoS One. 2014; 9: e99083. doi: 10.1371/journal.pone.0099083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zuo Y, Wang C, Sun X, Hu C, Liu J, Hong X, et al. Identification of matrix metalloproteinase-10 as a key mediator of podocyte injury and proteinuria. Kidney Int. 2021; 100: 837–849. doi: 10.1016/j.kint.2021.05.035 [DOI] [PubMed] [Google Scholar]
- 98.Rao VH, Meehan DT, Delimont D, Nakajima M, Wada T, Gratton MA, et al. Role for macrophage metalloelastase in glomerular basement membrane damage associated with alport syndrome. Am J Pathol. 2006; 169: 32–46. doi: 10.2353/ajpath.2006.050896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Naudin C, Smith B, Bond DR, Dun MD, Scott RJ, Ashman LK, et al. Characterization of the early molecular changes in the glomeruli of Cd151 -/- mice highlights induction of mindin and MMP-10. Sci Rep. 2017; 7: 15987. doi: 10.1038/s41598-017-15993-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Matsumoto S, Kobayashi T, Katoh M, Saito S, Ikeda Y, Kobori M, et al. Expression and localization of matrix metalloproteinase-12 in the aorta of cholesterol-fed rabbits: relationship to lesion development. Am J Pathol. 1998; 153: 109–119. doi: 10.1016/S0002-9440(10)65551-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res. 2006; 69: 562–573. doi: 10.1016/j.cardiores.2005.12.002 [DOI] [PubMed] [Google Scholar]
- 102.George B, Verma R, Soofi AA, Garg P, Zhang J, Park TJ, et al. Crk1/2-dependent signaling is necessary for podocyte foot process spreading in mouse models of glomerular disease. J Clin Invest. 2012; 122: 674–692. doi: 10.1172/JCI60070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Uchio K, Sawada K, Manabe N. Expression of macrophage metalloelastase (MMP-12) in podocytes of hereditary nephrotic mice (ICGN strain). J Vet Med Sci. 2009; 71: 305–312. doi: 10.1292/jvms.71.305 [DOI] [PubMed] [Google Scholar]
- 104.Shapiro SD, Griffin GL, Gilbert DJ, Jenkins NA, Copeland NG, Welgus HG, et al. Molecular cloning, chromosomal localization, and bacterial expression of a murine macrophage metalloelastase. J Biol Chem. 1992; 267: 4664–4671. doi: 10.1016/S0021-9258(18)42885-2 [DOI] [PubMed] [Google Scholar]
- 105.Shapiro SD, Kobayashi DK, Ley TJ. Cloning and characterization of a unique elastolytic metalloproteinase produced by human alveolar macrophages. J Biol Chem. 1993; 268: 23824–23829. doi: 10.1016/S0021-9258(20)80459-1 [DOI] [PubMed] [Google Scholar]
- 106.Rheault MN, Kren SM, Thielen BK, Mesa HA, Crosson JT, Thomas W, et al. Mouse model of X-linked Alport syndrome. J Am Soc Nephrol. 2004; 15: 1466–1474. doi: 10.1097/01.ASN.0000130562.90255.8F [DOI] [PubMed] [Google Scholar]
- 107.Sasaki H, Marusugi K, Kimura J, Kitamura H, Nagasaki K, Torigoe D, et al. Genetic background-dependent diversity in renal failure caused by the tensin2 gene deficiency in the mouse. Biomed Res. 2015; 36: 323–330. doi: 10.2220/biomedres.36.323 [DOI] [PubMed] [Google Scholar]
- 108.Nishino T, Sasaki N, Nagasaki K, Ahmad Z, Agui T. Genetic background strongly influences the severity of glomerulosclerosis in mice. J Vet Med Sci. 2010; 72: 1313–1318. doi: 10.1292/jvms.10-0144 [DOI] [PubMed] [Google Scholar]
- 109.Nishino T, Sasaki N, Nagasaki K, Ichii O, Kon Y, Agui T. The 129 genetic background affects susceptibility to glomerulosclerosis in tensin2-deficient mice. Biomed Res. 2012; 33: 53–56. doi: 10.2220/biomedres.33.53 [DOI] [PubMed] [Google Scholar]
- 110.Takeda Y, Kazarov AR, Butterfield CE, Hopkins BD, Benjamin LE, Kaipainen A, et al. Deletion of tetraspanin Cd151 results in decreased pathologic angiogenesis in vivo and in vitro. Blood. 2007; 109: 1524–1532. doi: 10.1182/blood-2006-08-041970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wright MD, Geary SM, Fitter S, Moseley GW, Lau LM, Sheng KC, et al. Characterization of mice lacking the tetraspanin superfamily member CD151. Mol Cell Biol. 2004; 24: 5978–5988. doi: 10.1128/MCB.24.13.5978-5988.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Andrews KL, Mudd JL, Li C, Miner JH. Quantitative trait loci influence renal disease progression in a mouse model of Alport syndrome. Am J Pathol. 2002; 160: 721–730. doi: 10.1016/S0002-9440(10)64892-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Takemon Y, Wright V, Davenport B, Gatti DM, Sheehan SM, Letson K, et al. Uncovering Modifier Genes of X-Linked Alport Syndrome Using a Novel Multiparent Mouse Model. J Am Soc Nephrol. 2021; 32: 1961–1973. doi: 10.1681/ASN.2020060777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Falcone S, Wisby L, Nicol T, Blease A, Starbuck B, Parker A, et al. Modification of an aggressive model of Alport Syndrome reveals early differences in disease pathogenesis due to genetic background. Sci Rep. 2019; 9: 20398. doi: 10.1038/s41598-019-56837-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kang JS, Wang XP, Miner JH, Morello R, Sado Y, Abrahamson DR, et al. Loss of α3/α4(IV) collagen from the glomerular basement membrane induces a strain-dependent isoform switch to α5α6(IV) collagen associated with longer renal survival in Col4a3-/- Alport mice. J Am Soc Nephrol. 2006; 17: 1962–1969. doi: 10.1681/ASN.2006020165 [DOI] [PubMed] [Google Scholar]
- 116.Sasaki H, Kimura J, Nagasaki K, Marusugi K, Agui T, Sasaki N. Mouse chromosome 2 harbors genetic determinants of resistance to podocyte injury and renal tubulointerstitial fibrosis. BMC Genet. 2016; 17: 69. doi: 10.1186/s12863-016-0378-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sasaki H, Sasaki N, Nishino T, Nagasaki K, Kitamura H, Torigoe D, et al. Quantitative trait Loci for resistance to the congenital nephropathy in tensin 2-deficient mice. PLoS One. 2014; 9: e99602. doi: 10.1371/journal.pone.0099602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Dalla Vestra M, Saller A, Mauer M, Fioretto P. Role of mesangial expansion in the pathogenesis of diabetic nephropathy. J Nephrol. 2001; 14:(Suppl 4): S51–S57. [PubMed] [Google Scholar]
- 119.Takahashi Y, Sasaki H, Okawara S, Sasaki N. Genetic loci for resistance to podocyte injury caused by the tensin2 gene deficiency in mice. BMC Genet. 2018; 19: 24. doi: 10.1186/s12863-018-0611-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Freedman BI, Beck SR, Rich SS, Heiss G, Lewis CE, Turner S, et al. A genome-wide scan for urinary albumin excretion in hypertensive families. Hypertens (Dallas, Tex 1979). 2003; 42: 291–296. [DOI] [PubMed] [Google Scholar]
- 121.Wellcome Trust Sanger Institute. Sanger SNP and Indel Data, 89+ Million Locations, 37 Inbred Strains of Mice. MPD:Sanger4. Mouse Phenome Database web Resour. (RRIDSCR_003212), Jackson Lab. Bar Harb. Maine USA 2017. https://phenome.jax.org (accessed January 10, 2022).
- 122.Harris EN, Cabral F. Ligand Binding and Signaling of HARE/Stabilin-2. Biomolecules. 2019; 9: 273. doi: 10.3390/biom9070273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hirose Y, Saijou E, Sugano Y, Takeshita F, Nishimura S, Nonaka H, et al. Inhibition of Stabilin-2 elevates circulating hyaluronic acid levels and prevents tumor metastasis. Proc Natl Acad Sci USA. 2012; 109: 4263–4268. doi: 10.1073/pnas.1117560109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Schledzewski K, Géraud C, Arnold B, Wang S, Gröne HJ, Kempf T, et al. Deficiency of liver sinusoidal scavenger receptors stabilin-1 and -2 in mice causes glomerulofibrotic nephropathy via impaired hepatic clearance of noxious blood factors. J Clin Invest. 2011; 121: 703–714. doi: 10.1172/JCI44740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Maeda-Smithies N, Hiller S, Dong S, Kim HS, Bennett BJ, Kayashima Y. Ectopic expression of the Stabilin2 gene triggered by an intracisternal A particle (IAP) element in DBA/2J strain of mice. Mamm Genome. 2020; 31: 2–16. doi: 10.1007/s00335-019-09824-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kayashima Y, Makhanova NA, Matsuki K, Tomita H, Bennett BJ, Maeda N. Identification of aortic arch-specific quantitative trait loci for atherosclerosis by an intercross of DBA/2J and 129S6 apolipoprotein E-deficient mice. PLoS One. 2015; 10: e0117478. doi: 10.1371/journal.pone.0117478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kaul A, Singampalli KL, Parikh UM, Yu L, Keswani SG, Wang X. Hyaluronan, a double-edged sword in kidney diseases. Pediatr Nephrol. 2022; 37: 735–744. doi: 10.1007/s00467-021-05113-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Collum SD, Chen NY, Hernandez AM, Hanmandlu A, Sweeney H, Mertens TCJ, et al. Inhibition of hyaluronan synthesis attenuates pulmonary hypertension associated with lung fibrosis. Br J Pharmacol. 2017; 174: 3284–3301. doi: 10.1111/bph.13947 [DOI] [PMC free article] [PubMed] [Google Scholar]