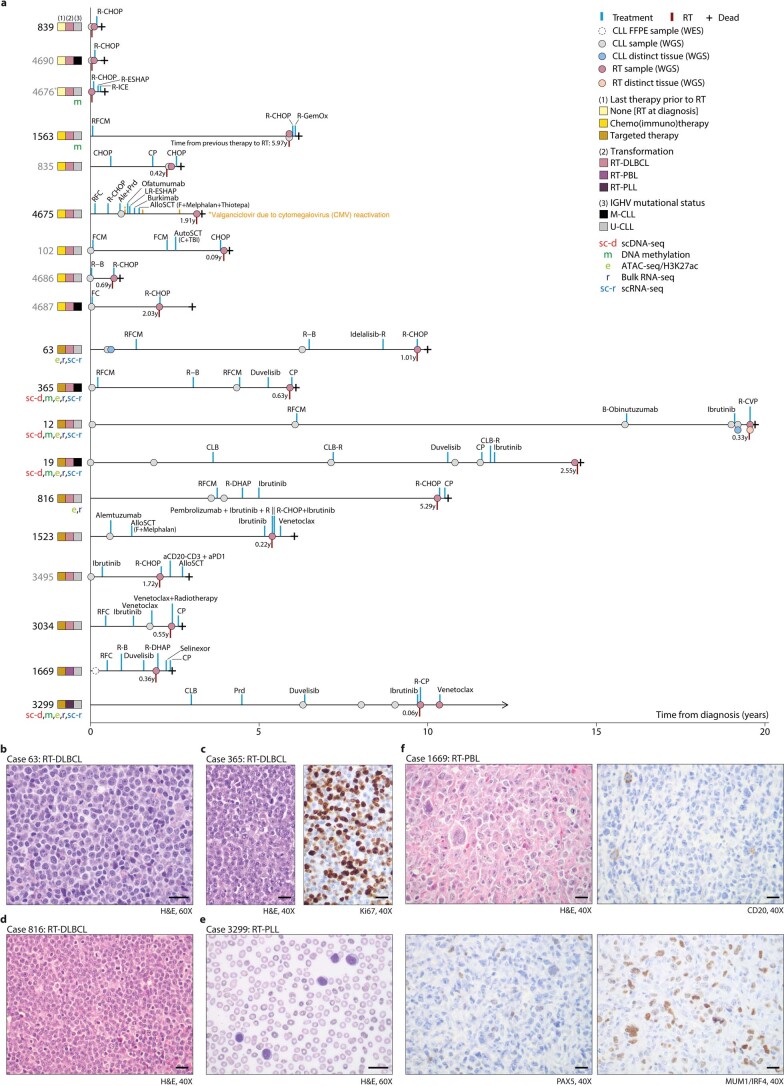
Extended Data Fig. 1. Cohort studied and types of Richter transformation.
a. Representation of the disease course of the patients included in the study. Each sample analyzed, treatment and date of RT are depicted. Patients labeled in gray lacked germline DNA. Patient 4676 also lacked DNA from the previous CLL sample. Patients are grouped based on the last line of therapy received before RT in three groups: patients developing RT before any treatment, after chemo(immuno)therapy, and after targeted therapy. The type of transformation (RT-DLBCL, diffuse large B cell lymphoma type; RT-PLL, prolymphocytic transformation; RT-PBL, plasmablastic transformation) and IGHV mutational status are also shown. Additional molecular studies conducted in each case are also depicted. Abbreviations: Ale: alemtuzumab; AlloSCT: allogenic stem-cell transplantation; AutoSCT: autologous stem-cell transplantation; B: bendamustine; Burkimab: rituximab, methotrexate, dexametasone, ifosfamide, vincristine, etoposide, cytarabine, doxorubicin and vindesine; C: cyclophosphamide; CHOP: cyclophosphamide, doxorubicin, vincristine and prednisone; CLB: chlorambucil; CLB-R: chlorambucil and rituximab; CP: cyclophosphamide and prednisone; F: fludarabine; FCM: fludarabine, cyclophosphamide and mitoxantrone; G-GemOx: rituximab, gemcitabine, and oxaliplatin; LR-ESHAP: lenalidomide, rituximab, etoposide, methyl-prednisolone, cytarabine and cisplatin; M: mitoxantrone; Prd: prednisone; R: rituximab; R-B: rituximab and bendamustine; R-CHOP: rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone; R-CVP: rituximab, cyclophosphamide, vincristine and prednisone; R-DHAP: rituximab, dexamethasone, cytarabine and cisplatin; R-ESHAP: rituximab, etoposide, methyl-prednisolone, cytarabine and cisplatin; RFC: fludarabine, cyclophosphamide and rituximab; RFCM: rituximab, fludarabine, cyclophosphamide and mitoxantrone; R-ICE: rituximab, ifosfamide, carboplatin and etoposide; TBI: total body irradiation. b. Morphology of the RT-DLBCL of patient 63 (hematoxylin-eosin, H&E, staining). c. Morphology of the RT-DLBCL of patient 365 and Ki67 staining showing high proliferative index. d. Morphology of the RT-DLBCL of patient 816. e. Morphology of the RT-PLL of patient 3299. f. Morphology of the RT-PBL of patient 1669 (H&E staining), which was negative for CD20 and PAX5, while positive for MUM1/IRF4. Each experiment for b-f was repeated twice. The scale bars in b-f represents 20 μm.