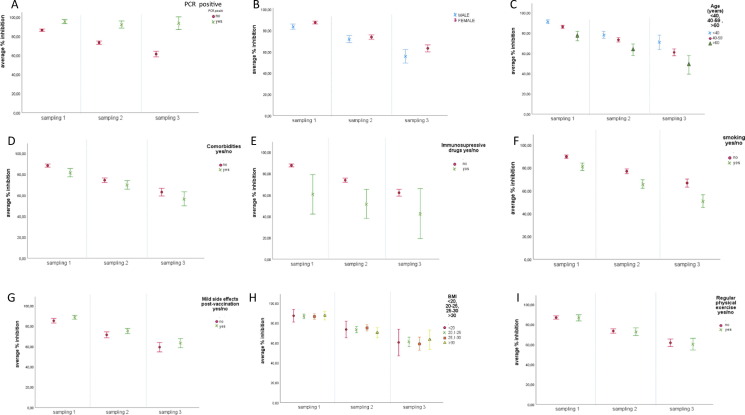
Fig. 2.
Mean inhibition rates per time sampling1 and: (A) SARS-CoV2 infection: no past SARS-CoV-2 infection and
no past SARS-CoV-2 infection and past SARS-CoV-2 infection;(B) gender:
past SARS-CoV-2 infection;(B) gender: male and
male and female;(C) age:
female;(C) age: < 40 years old,
< 40 years old, 40-59 years old and
40-59 years old and > 60 years old;(D) comorbidities:
> 60 years old;(D) comorbidities: no,
no, yes;(E) use of immunosuppressive drugs:
yes;(E) use of immunosuppressive drugs: no,
no, yes;(F) tobacco use:
yes;(F) tobacco use: no,
no, yes;(G) mild adverse events post vaccination:
yes;(G) mild adverse events post vaccination: no,
no, yes;(H) BMI:
yes;(H) BMI: < 20,
< 20, 20.1–25,
20.1–25, 25.1–30 and
25.1–30 and > 30;(I) regular physical exercise:
> 30;(I) regular physical exercise: no,
no, yes.
yes.
Sampling 1: samples collected approximately 3 months after the second vaccine dose (mean: 95.3 days, range: 52–135 days). Sampling 2: samples collected approximately 6 months after the second vaccine dose (mean: 184.6 days, range: 136–225 days).Sampling 3: samples collected approximately 9 months after the second vaccine dose (mean: 262.9 days, range: 226–322 days). Error Bars 95% CI SARS-CoV-2: severe acute respiratory syndrome coronavirus 2; BMI: body mass index; CI: confidence interval