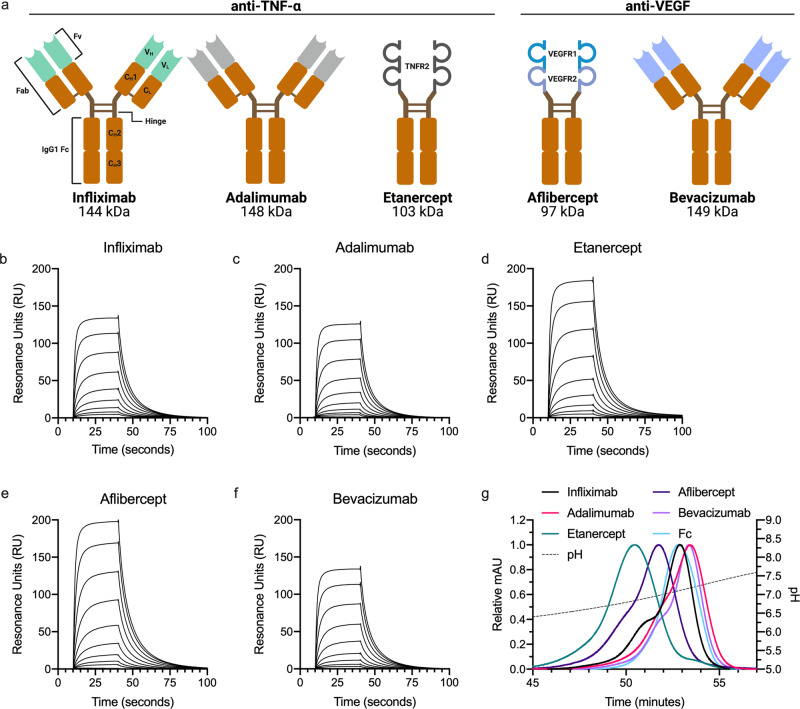
Fig. 3. Fc-fused modalities affect FcRn-binding throughout the pH-gradient.
a Schematic illustration of structural elements in the ABT panel. All five ABTs contain an IgG1 Fc (orange, CH2 and CH3). ABMs (Fvs and receptor domains) are marked in light green, gray or shades of blue. Infliximab is a chimeric mouse-derived human IgG1 with a murine Fv (marked in light green) binding to TNF-a. Adalimumab is a fully human IgG1 binding to TNF-a (Fv gray). Etanercept consists of the extracellular domains of TNFR2 (gray) fused to the IgG1 Fc via a hinge region. Aflibercept consists of D2 of VEGFR1 (turquoise) linked to D3 of VEGFR2 (light blue) fused to an IgG1 Fc. Bevacizumab is a fully human IgG1 binding to active isotypes of VEGF (Fv light blue). MW of the total amino acid sequence of each ABT is indicated. b–f Representative SPR sensorgrams of titrated amounts of monomeric FcRn injected over immobilized ABTs (~400 RU). g Elution profiles of ABTs from FcRn chromatography shown as relative fluorescence intensity throughout the pH gradient. The pH is plotted on the right Y-axis and indicated by a stapled line. Color labeling; infliximab (black), adalimumab (pink), etanercept (teal), aflibercept (dark purple), bevacizumab (light purple), Fc (light blue).