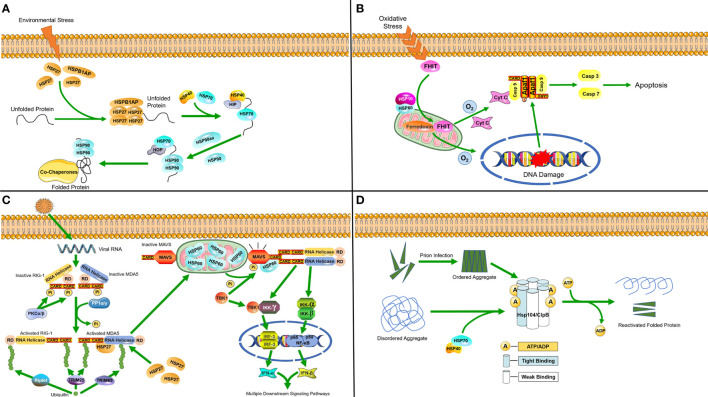
Figure 1.
Molecular mechanisms of heat shock proteins induced under stress (A) Hsp27 binds to unfolded proteins that accumulate in the cytosol during stress, and then diverts unfolded proteins along the protein folding pathway, ultimately reaching HSP90. (B) In response to oxidative stress, the HSP60-10 complex helps to localize FHIT protein to the mitochondria, where it stabilizes ferredoxin reductase, leading to enhanced production of reactive oxygen species. This in turn triggers cytochrome c release and subsequent activation of the caspase cascade, ultimately causing apoptosis. (C) Following viral invasion, the RLR/MDA5 signaling pathway is activated. HSP27 can specifically stabilize MDA5 during expression to enhance the RLR/MDA5 signaling pathway. In mitochondria, HSP60 interacts with MAVS to increase MAVS-mediated IFN-β promoter activity and the transcriptional levels of IFN-β. Furthermore, it can upregulate MAVS-induced mRNA transcription of IFN-stimulated genes (ISGs). (D) Hsp104/ClpB complexes in host cells process disordered aggregates accumulated following cellular stress as well as ordered aggregates formed after prion infection with the help of the HSP70-40 partner system, dissociating them into component proteins and reactivating them.