Abstract
Background
Accumulating evidence has revealed that the gut microbiota influences the effectiveness of immune checkpoint inhibitors (ICIs) in cancer patients. As a part of the human microbiome, Helicobacter pylori (H. pylori) was reported to be associated with reduced effectiveness of anti-PD1 immunotherapy in patients with non-small-cell lung cancer (NSCLC). Gastric cancer is more closely related to H. pylori, so we conducted a retrospective analysis to verify whether the association of H. pylori and effectiveness is applicable to advanced gastric cancer (AGC) patients.
Material and methods
AGC patients who had evidence of H. pylori and received anti-PD-1 antibodies were enrolled in the study. The differences in the disease control rate (DCR), overall survival (OS) and progression-free survival (PFS) between the H. pylori-positive group and the negative group were compared.
Results
A total of 77 patients were included in this study; 34 patients were H. pylori positive, and the prevalence of H. pylori infection was 44.2%. Compared with the H. pylori-negative group, patients in the H. pylori-positive group had a higher risk of nonclinical response to anti-PD-1 antibody, with an OR of 2.91 (95% CI: 1.13–7.50). Patients in the H. pylori-negative group had a longer OS and PFS than those in the positive group, with an estimated median OS of 17.5 months vs. 6.2 months (HR = 2.85, 95% CI: 1.70–4.78; P = 0.021) and a median PFS of 8.4 months vs. 2.7 months (HR = 3.11, 95% CI: 1.96–5.07, P = 0.008). Multivariate analysis indicated that H. pylori infection was independently associated with PFS (HR = 1.90, 95% CI: 1.10–3.30; P = 0.022).
Conclusion
Our study unveils for the first time that H. pylori infection is associated with the outcome of immunotherapy for AGC patients. Multicenter, large sample and prospective clinical studies are needed to verify the association.
Keywords: Advanced gastric cancer, Immune checkpoint inhibitors, Helicobacter pylori, Prognosis
Introduction
Gastric cancer is the fifth most common cancer and third most common cause of cancer-related death worldwide after lung and colorectal cancers [1]. Although improvements in survival trends have occurred in gastric cancer patients diagnosed with localized disease, the prognosis of advanced gastric cancer (AGC) is still poor. Epidemiological data showed that the five-year survival rates for gastric cancer patients diagnosed with distant disease remain less than 5% [2]. The poor efficacy and serious adverse reactions of traditional chemotherapy and the small proportion of Her-2-positive patients limit the application of targeted therapy in gastric cancer. In the last decade, immune checkpoint inhibitors (ICIs) have emerged as an exciting treatment strategy across a spectrum of malignancies. This includes monoclonal antibodies that inhibit programmed cell death protein 1 (PD-1), programmed cell death ligand 1 (PD-L1) and cytotoxic T-lymphocyte antigen 4 (CTLA-4). Although ICIs are used for the treatment of AGC, they are not promising in most cases [3]. Even for patients with positive PD-L1 expression, the effective rate of pembrolizumab is only 16%, and neither progression-free survival (PFS) nor overall survival (OS) is significantly prolonged when compared with paclitaxel [4]. Therefore, it is critical to find more practical prognostic markers to screen the most suitable population.
Accumulating evidence has revealed that the gut microbiota has a considerable influence on the effectiveness of checkpoint blockade immunotherapy in human cancer patients [5–7], and the use of antibiotics inhibits the clinical benefit of ICIs in patients with advanced cancer [8, 9]. Interestingly, some gut microbiota have been found to be biomarkers of nonresponsiveness to ICIs [5, 10], while some of them are associated with an effective therapeutic response [11, 12]. As a part of the human microbiome, Helicobacter pylori (H. pylori) is one of the most widespread bacterial pathogens worldwide, with an approximately 50% prevalence in the global population. Chronic infection with H. pylori is the main cause of gastric cancer, accounting for approximately 89% of distal gastric cancer cases worldwide [13, 14]. Therefore, it has been categorized as a class 1 carcinogen by the World Health Organization [15]. H. pylori colonizes the surface of the stomach mucosa and is not only closely associated with many disorders of the upper gastrointestinal tract but also related to some diseases localized outside the stomach [16–18]. In contrast, some diseases, such as asthma, allergies, inflammatory bowel disease, and esophageal eosinophilia, are inversely associated with H. pylori infection [19–21]. These are all systemic inflammatory diseases related to disorders of the immune state of the body. These clinical phenomena suggest that H. pylori may alter the balance of immunomodulation. Therefore, it is reasonable to speculate that H. pylori may influence the response to cancer immunotherapies. However, there are very few clinical studies in this area, and thus far, we have not seen any relevant reports on the relationship between H. pylori and immunotherapy of gastric cancer.
In this study, we compared the differences in disease control rate (DCR), OS and PFS between the H. pylori-positive group and the H. pylori-negative group to evaluate the association of H. pylori infection with outcomes in AGC patients treated with anti-PD-1 antibodies.
Materials
Patients
This was a retrospective study conducted with a cohort of AGC patients treated with ICIs at General Hospital of Chinese PLA between May 2015 and June 2020. The inclusion criteria were as follows: 1) patients with pathologically or cytologically confirmed AGC, including cardia and noncardia gastric cancer; 2) patients treated with anti-PD-1 antibody or CTLA-4 antibody; 3) all patients with evidence of H. pylori examination. Exclusion criteria: Subjects received only one cycle of ICIs therapy, and clinical data were not available or were lost to follow-up.
H. pylori status
To avoid the effect of anti-PD-1 antibody or CTLA-4 antibody on H. pylori examination, all patients should be tested for H. pylori prior to ICIs initiation. The diagnostic methods for H. pylori infection include the 13C-urea breath test (13C-UBT), H. pylori stool antigen (HpSA) test and histopathology. 13C-UBT was performed in the morning after fasting for at least 8 h. Breath samples were collected from each subject at baseline and 30 min after drinking 70 mL of water containing 75 mg of 13C-urea. An additional breath sample was collected 30 min after the ingestion of the tracer. The test was performed with a 13C-breath test instrument (Fischer Analysen Instrumente GmbH, Leipzig, Germany). The results were defined as positive when the delta over baseline (DOB) was > 4‰. Fresh stool samples were used for the HpSA test. According to the manufacturer’s instructions, a one-step chromatographic immunoassay known as the CerTest H. pylori Blister Test (CerTest Biotec S.L.) was applied for the analysis. Based on the condition of the control line and sample line, the samples were divided into three types: positive, negative and intermediate. All intermediate data were excluded from the analysis. The endoscopic diagnosis and pathological diagnosis of H. pylori were performed by experienced endoscopy doctors and pathologists, respectively.
Data collection and evaluation
The following data of subjects were collected from the medical records: age, sex, Eastern Cooperative Oncology Group Performance Status (ECOG PS), pathological type, primary tumor site, tumor differentiation, treatment line, anti-PD-1 agent, response rate and so on. All procedures performed in this study involving human participants were in accordance with the ethical standards of the national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards (Medical Ethics Committee of PLA General Hospital No. S2019-136–01).
Tumor assessment was performed at baseline and then after every two treatment cycles, which was generally after every 6 weeks. According to the Response Evaluation Criteria in Solid Tumors (RECIST) guidelines (version 1.1), clinical responses were categorized as complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD). The DCR was defined as patients with CR, PR, or SD. OS was calculated from the date of first immunotherapy administration until death due to any cause or up to the end of the follow-up. PFS HR was defined as the time from the date of first immunotherapy administration to the date of disease progression or death due to any cause before progression. OS and PFS that were not reached were considered censored data. The line of immunotherapy for AGC patients was classified as follows: ICIs was included in the first antitumor therapy after the diagnosis of advanced gastric cancer (lines of immunotherapy < 2), otherwise it’s defined as non-first-line therapy (lines of immunotherapy ≥ 2).In the absence of clinical progression of disease, if other anti-cancer agent that is part of a systemic anti-cancer therapy is discontinued due to toxicity and substituted by another anti-cancer agent of the same class, retain the same line of therapy. Irrespective of clinical progression of disease, if the dose or schedule of administration of one or more anti-cancer agent of an ongoing systemic anti-cancer therapy is modified for any reason, retain the same line of therapy.The differences in DCR, OS and PFS between the H. pylori-positive group and the H. pylori-negative group were compared.
Statistical analysis
Categorical data are expressed as numbers and percentages, and the groups were compared using the chi-squared test. Survival curves for each group were estimated using Kaplan–Meier curves and compared by the log-rank test. A Cox proportional risk regression model was used for the multivariate analysis. The results are presented as hazard ratios (HR) with 95% confidence intervals (CIs). Two-sided P value < 0.05 was considered statistically significant. GraphPad Prism 7.0 (GraphPad Software, La Jolla, CA, USA) and SPSS 22.0 (IBM Corp., Armonk, NY, USA) were used for statistical analysis.
Results
Clinical characteristics of patients
A total of 95 patients were available in this study. Among them, 5 patients received only one cycle of ICIs therapy, 6 patients were lost to follow-up and 3 patients with incomplete outcome data.The H. pylori results were not reliable in four patients. Finally, a total of 77 patients were included in this study with a median age of 58.0 years (range: 24–88). Among them, 54 patients (70.1%) were male, and 23 patients (29.9%) were female. The ECOG PS was 0–1 for 62 patients (80.5%), and 66 patients (85.7%) were stage IV when they were diagnosed with gastric cancer. Regarding the histopathology type, 73 patients (94.8%) had adenocarcinoma, 1 patient (1.3%) had squamous carcinoma, and 3 patients (3.9%) had carcinoid carcinoma. In 15 patients (19.5%), the primary site was the gastroesophageal junction, and in 62 patients (80.5%), the primary site was the stomach. The anti-PD-1 antibodies used were nivolumab (43 patients, 55.8%), pembrolizumab (29 patients, 37.7%), and camrelizumab/toripalimab/tislelizumab (5 patients, 6.5%). Forty-four patients (57.1%) had undergone gastrectomy before immunotherapy, and 53 patients received immunotherapy in combination with other treatments, including chemotherapy and targeted therapy. Among these 77 patients, 60 patients received the HpSA test, 11 patients received 13C UBT, and 6 patients were diagnosed by histopathology. The prevalence of H. pylori infection was 44.2% in this cohort. The baseline characteristics and response rate are summarized in Table 1.
Table 1.
General characteristics of participants and response rate according to H. pylori status
| Variable | Total (n,%) |
H. pylori negative (n,%) |
H. pylori positive (n,%) |
P value | |
|---|---|---|---|---|---|
| Gender | Male | 54(70.1) | 31(72.1) | 23(67.6) | 0.672 |
| Female | 23(29.9) | 12(27.9) | 11(32.4) | ||
| Age (years) | < 65 | 58(75.3) | 30(69.8) | 28(82.4) | 0.203 |
| ≥ 65 | 19(24.7) | 13(30.2) | 6(17.6) | ||
| ECOG PS | 0–1 | 62(80.5) | 38(88.4) | 24(70.6) | 0.050 |
| ≥ 2 | 15(19.5) | 5(11.6) | 10(29.4) | ||
| Stage | III | 11(14.3) | 4(9.3) | 7(20.6) | 0.281 |
| IV | 66(85.7) | 39(90.7) | 27(79.4) | ||
| Histology | Adenocarcinoma | 73(94.8) | 39(90.7) | 34(100.0) | 0.089 |
| Squamous | 1(1.3) | 1(2.3) | 0 | ||
| Carcinoid | 3(3.9) | 3(7.0) | 0 | ||
| Primary tumor site | Gastro-esophageal unction | 15(19.5) | 11(25.6) | 4(11.8) | 0.128 |
| Stomach | 62(80.5) | 32(74.4) | 30(88.2) | ||
| Tumor differentiation | Undifferentiation | 2(2.6) | 0 | 2(5.9) | 0.156 |
| Poor differentiation | 66(85.7) | 37(86.0) | 29(85.3) | ||
| Median/ High differentiation | 9(11.7) | 6(14.0) | 3(8.8) | ||
| Her-2 expression | Negative | 36(46.8) | 17(39.5) | 19(55.9) | 0.169 |
| Positive | 19(24.7) | 14(32.6) | 5(14.7) | ||
| Not examined | 22(28.6) | 12(27.9) | 10(29.4) | ||
| PD-L1 expression | Negative | 17(32.1) | 8(18.6) | 9(26.5) | 0.316 |
| Positive | 8(10.4) | 3(7.0) | 5(14.7) | ||
| Not examined | 52(67.5) | 32(74.4) | 20(58.8) | ||
| Surgery | No | 33(42.9) | 16(37.2) | 17(50.0) | 0.260 |
| Yes | 44(57.1) | 27(62.8) | 17(50.0) | ||
| Drugs of ICIs | Nivolumab | 43(55.8) | 24(55.8) | 19(55.9) | 0.980 |
| Pembrolizumab | 29(37.7) | 16(37.2) | 13(38.2) | ||
| Camrelizumab/Toripalimab/Tislelizumab | 5(6.5) | 3(7.0) | 2(5.9) | ||
| Lines of immunotherapy | < 2 | 22(28.6) | 15(34.9) | 7(20.6) | 0.168 |
| ≥ 2 | 55(71.4) | 28(65.1) | 27(79.4) | ||
| Combined with other therapies | No | 24(31.2) | 10(23.3) | 14(41.2) | 0.092 |
| Yes | 53(68.8) | 33(76.7) | 20(58.8) | ||
| Response rate | CR/PR | 14(18.2) | 12(27.9) | 2(5.9) | |
| SD | 33(42.9) | 19(44.2) | 14(41.2) | ||
| PD | 30(39.0) | 12(27.9) | 18(52.9) | ||
| DCR (CR/PR + SD) | 47(61.0) | 31(72.1) | 16(47.1) | 0.027* | |
ECOG PS Eastern Cooperative Oncology Group Performance Status, PD-1 Programmed cell death 1, ICIs Immune checkpoint inhibitors, CR Complete response, PR Partial response, SD Stable disease, PD Progressive disease, DCR Disease control rate
*, P < 0.05 indicates statistical significance
Association between the H. pylori status and effectiveness of anti-PD1 immunotherapy
The optimal efficacy was evaluated for all patients. Of the 43 patients in the H. pylori-negative group, 12 patients experienced PD by the end of follow-up, while among the 34 patients in the positive group, 18 patients had PD. The DCR in the negative group and positive group were 72.1% and 47.1%, respectively (P = 0.027). Compared with the H. pylori-negative group, patients in the H. pylori-positive group had a higher risk of nonclinical response to anti-PD-1 antibody, with an OR of 2.91 (95% CI: 1.13–7.50) (Table 2).
Table 2.
Efficacy and prognosis based on the H. pylori status
| H. pylori status | Response rate | OS (months) | PFS (months) | ||||
|---|---|---|---|---|---|---|---|
| DCR (%) | OR(95%CI) | Median | HR(95%CI) | Median | HR(95%CI) | ||
| negative | (n = 43) | 72.1 | 1 [Reference] | 17.5 | 1 [Reference] | 8.4 | 1 [Reference] |
| positive | (n = 34) | 47.1 | 2.91(1.13–7.50) | 6.2 | 2.85(1.70–4.78) | 2.7 | 3.11(1.96–5.07) |
| P value | 0.027* | 0.021* | 0.008* | ||||
DCR Disease control rate, OR Odd ratio, HR Hazards ratio, CI Confidence interval, OS Overall survival, PFS Progression free survival
*, P < 0.05 indicates statistical significance
Association between the H. pylori status and prognosis of anti-PD1 immunotherapy
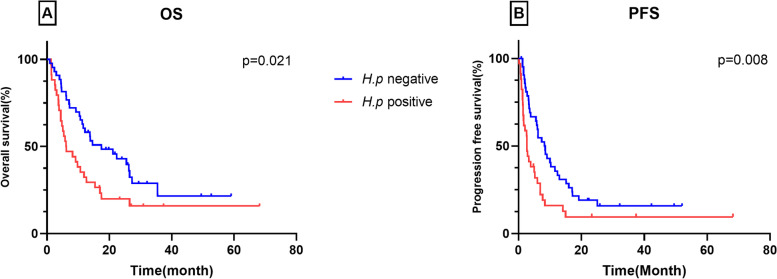
Of the 77 patients, 57 patients (78.3%) died within the follow-up period. The median OS and PFS were 11.6 months (95% CI: 7.2–15.4) and 5.2 (95% CI: 3.2–6.9) months, respectively. Patients in the H. pylori-negative group had a longer OS than those in the positive group, with an estimated median survival of 17.5 months vs. 6.2 months (HR = 2.85, 95% CI: 1.70–4.78; P = 0.021). A similar prognostic association was observed for PFS: we observed prolonged PFS in patients in the H. pylori-negative group compared to the positive group (8.4 months vs. 2.7 months, HR = 3.11, 95% CI: 1.96–5.07, P = 0.008; Table 2). The survival curves for these two groups are presented in Fig. 1. Cox regression showed that H. pylori infection was independently associated with PFS (HR = 1.90, 95% CI: 1.10–3.30; P = 0.022). Although there was a correlation between H. pylori infection and OS (HR = 1.76, 95% CI: 0.99–3.12), it was not statistically significant (P = 0.052) (Table 3).
Fig. 1.
Survival curves of overall survival (OS) and progression-free survival (PFS) of patients with AGC treated with anti-PD-1 antibodies
Table 3.
Multivariate analysis for overall survival and progression-free survival in the pooled cohort
| Overall Survival | Progression-free Survival | |||
|---|---|---|---|---|
| Variable | HR (95%CI) | P value | HR (95%CI) | P value |
| Gender | 0.096 | |||
| Male | 1[Reference] | 0.999 | 1[Reference] | |
| Female | 1.00(0.56–2.02) | 0.60(0.33–1.10) | ||
| Age (years) | 0.858 | 0.395 | ||
| < 65 | 1[Reference] | 1[Reference] | ||
| ≥ 65 | 1.06(0.56–2.02) | 0.76(0.40–1.44) | ||
| ECOG PS | 0.001* | 0.003* | ||
| 0–1 | 1[Reference] | 1[Reference] | ||
| ≥ 2 | 3.49(1.64–7.44) | 2.82(1.43–5.58) | ||
| Primary tumor site | 0.885 | 0.628 | ||
| Gastro-esophageal unction | 1[Reference] | 1[Reference] | ||
| Stomach | 0.95(0.47–1.94) | 0.86(0.46–1.61) | ||
| Stage | 0.129 | 0.134 | ||
| III | 1[Reference] | 1[Reference] | ||
| IV | 2.05(0.81–5.16) | 1.90(0.82–4.37) | ||
| Lines of immunotherapy | 0.040* | 0.012* | ||
| < 2 | 1[Reference] | 1[Reference] | ||
| ≥ 2 | 2.00(1.03–3.90) | 2.22(1.19–4.12) | ||
| Combined with other therapies | 0.041* | 0.148 | ||
| No | 1[Reference] | 1[Reference] | ||
| Yes | 0.55(0.31–0.98) | 0.69(0.41–1.14) | ||
| H. pylori status | 0.052 | 0.022* | ||
| Negative | 1[Reference] | 1[Reference] | ||
| Positive | 1.76(0.99–3.12) | 1.90(1.10–3.30) | ||
ECOG PS Eastern Cooperative Oncology Group Performance Status, HR Hazards ratio, CI Confidence interval
*, P < 0.05 indicates statistical significance
Discussion
The human microbiome includes the microbes that reside in the human body, such as bacteria, viruses, fungi, and protozoa, and their genomes. Currently, a growing number of studies have evidenced the relationship between the local resident microbiota and the gut microbiome in cancer and cancer treatment [22–24]. The utilization of ICIs is considered a revolution in cancer therapy that changes the poor prognosis of many advanced-stage cancers, and the tumor response to ICIs was found to have a strong association with gut microbiota in both clinical cohorts and preclinical mouse models [25–29]. Several studies have found that Bacteroidetes may be a biomarker of nonresponse to ICIs in patients with metastatic melanoma [10, 12]. In non-small-cell lung cancer (NSCLC) and renal cell carcinoma (RCC), Akkermansia muciniphila and Alistipes have been found to be biomarkers of ICI responders [6]. In addition, the gut microbiota also has an impact on the survival of tumor patients. Routy et al. found that among patients with NSCLC, RCC, and urothelial carcinoma (UC) who received anti-PD1 immunotherapy, the PFS and OS were significantly reduced in patients treated with antibiotics [6]. These studies indicate that there is a strong correlation between gut microbiota and the efficacy of tumor immunotherapy. However, it is well known that the gut microbiome is a very complex system, so studying the effects of single bacteria on tumor immunotherapy has inevitable limitations, and the small intestine and stomach microbiota may also influence the effectiveness of ICIs [30].
Similar to the intestinal flora, the relationship between H. pylori infection and tumor immunotherapy has attracted the attention of researchers. Recently, Oster P et al. found that in mice engrafted with MC38 colon adenocarcinoma or B16-OVA melanoma cells, the tumor volumes of noninfected mice undergoing anti-CTLA4 and/or PD-1 or anticancer vaccine treatments were significantly smaller than those of infected mice. Two independent cohorts of patients with NSCLC on anti-PD-1 therapy verified that H. pylori seropositivity is associated with a lower effectiveness of anti-PD-1 immunotherapy in humans [31]. Their study is the first to suggest that the stomach microbiota affects the response to cancer immunotherapies. Similarly, our research found that H. pylori-positive patients had a higher risk of nonclinical response to anti-PD-1 antibody, and we also observed prolonged PFS and OS in patients in the H. pylori-negative group compared to the H. pylori-positive group. To our knowledge, the present study is the first to evaluate the association of H. pylori infection with outcome in AGC patients treated with an anti-PD-1 antibody. Although Cox regression revealed that H. pylori infection was not independently associated with OS, it is still helpful to predict the efficacy and prognosis of immunotherapy for AGC patients. It should be noted that the diagnostic methods for H. pylori infection in this study include 13C-UBT, HpSA test and histopathology, which all reflect the current active H. pylori infection. This is different from the study of Oster P et al., in which the H. pylori seropositive patients included both past and current infection populations. Although they found that the eradication of H. pylori infection by antibiotic administration does not increase the efficacy of vaccine-based immunotherapy, we cannot exclude the possibility that current active H. pylori infection and past infection may have a different influence on tumor immunotherapy.
The treatment of tumors by immune checkpoint inhibitors depends on the activation of immune cells [32], but the reason why H. pylori infection can affect tumor immunotherapy is not clear. According to the current research, this may be attributed to the microenvironment. Like most solid tumors, the microenvironment of epithelial-derived gastric adenocarcinoma consists of a variety of stromal cell types, including fibroblasts and neuronal, endothelial and immune cells. It is reported that H. pylori infection can prevent allergic asthma in mouse models through the induction of regulatory T cells [33]. Oster P et al. found that H. pylori inhibited antitumoural CD8+ T-cell responses by altering the cross-presentation activities of dendritic cells (DCs) in humans. They also observed a decreased number of myeloid cells and a substantially decreased expression of genes induced by type I interferon, IFNγ and IL-6 in the tumors of infected patients with NSCLC undergoing anti-PD1 treatment [31]. It is possible that the effect of H. pylori and H. pylori-derived factors on immune cells influences the effect of tumor immunotherapy. However, the impact of H. pylori infection on the composition of human gastrointestinal microbiota has been verified [34], and it has been reported that the immunopathogenesis of the stomach induced by H. pylori could trigger large intestinal microbiota [35]. Therefore, it is reasonable for us to suspect that H. pylori, in addition to affecting immune cells, may alter the gastrointestinal microbiota to influence tumor immunotherapy. Additional experiments are needed to explore the underlying mechanisms of H. pylori in decreasing the effectiveness of cancer immunotherapies.
There were some limitations in this study. First, this was a retrospective analysis of a small sample size from a single center, so external validation cohort studies with larger sample sizes are needed to confirm the robustness of our findings.
Second, the diagnoses of H. pylori in this study reflect the current active infection. Further research is needed to determine whether this result is applicable to past infection populations. Finally, as important prognostic factors for gastric cancer immunotherapy, the data of PD-L1 combined positive score positivity, microsatellite instability-high (MSI-H) and Epstein–Barr virus-positive (EBV +) [36] were incomplete in this study, so they were not included in multivariate regression analysis.
In summary, our study is the first to show the association between H. pylori infection and the outcome of immunotherapy for AGC patients. In the future, H. pylori may become a powerful prognostic biomarker of personalized immunotherapy for cancer patients. However, multicenter, large sample and prospective clinical studies are needed to verify the association. The role of H. pylori in predicting prognosis in patients treated with anti-PD-1 antibody, and the underlying molecular mechanisms need further study.
Acknowledgements
We would like to thank Prof. Yi Hu at the Department of Medical Oncology, Chinese PLA General Hospital, China, for his help of this study.
Abbreviations
- AGC
Advanced gastric cancer
- ICIs
Immune checkpoint inhibitors
- PD-1
Programmed cell death protein 1
- PD-L1
Programmed cell death ligand 1
- CTLA-4
Cytotoxic T-lymphocyte antigen 4
- PFS
Progression-free survival
- OS
Overall survival
- H. pylori
Helicobacter pylori
- DCR
Disease control rate
- 13C-UBT
13C-urea breath test
- HpSA
H. pylori Stool antigen
- DOB
Delta over baseline
- ECOG PS
EASTERN Cooperative Oncology Group Performance Status
- RECIST
Response Evaluation Criteria in Solid Tumors
- CR
Complete response
- PR
Partial response
- SD
Stable disease
- PD
Progressive disease
- HR
Hazard ratios
- CIs
Confidence intervals
- NSCLC
Non-small-cell lung cancer
- RCC
Renal cell carcinoma
- UC
Urothelial carcinoma
- DCs
Dendritic cells
- MSI-H
Microsatellite instability-high
- EBV +
Epstein–Barr virus-positive
Authors’ contributions
Hebin Che and Qi Xiong performed the study and drafted the manuscript. Shixue Chen and Jinxia Ma participated in the clinical data collection and analyses. Jinxia Ma carried out the 13C-UBT and clinical tests. Huan Wu performed the statistical analysis. Hongli Xu designed the study, performed critical revision of the manuscript. Baicun Hou conceived of the study, participated in its coordination, and wrote the manuscript. Baicun Hou had primary responsibility for final content. All authors read and approved the submitted version.
Funding
None.
Availability of data and materials
The raw data supporting the conclusions of this article will be made available by the corresponding author Baicun Hou, upon reasonable request.
Declarations
Ethics approval and consent to participate
All procedures performed in this study involving human participants were in accordance with the ethical standards of the national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The Ethics Committee of PLA General Hospital (Beijing, China) approved the review of the medical records (approval number: S2019-136–01). Since this is a retrospective statistical analysis based on electronic health records collected as part of standard healthcare, without disclosing the patients’ identity, no individual patients were directly contacted for the data. The need of consent was waived by the institutional ethical committee of the Chinese PLA General Hospital.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Hebin Che and Qi Xiong contributed equally to this work and share first authorship.
Contributor Information
Hongli Xu, Email: xuhongli@301hospital.com.cn.
Baicun Hou, Email: 769646266@qq.com.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Thrift AP, El-Serag HB. Burden of gastric cancer. Clin Gastroenterol Hepatol. 2020;18(3):534–542. doi: 10.1016/j.cgh.2019.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xie J, Fu L, Jin L. Immunotherapy of gastric cancer: past, future perspective and challenges. Pathol Res Pract. 2021;218:153322. doi: 10.1016/j.prp.2020.153322. [DOI] [PubMed] [Google Scholar]
- 4.Shitara K, Ozguroglu M, Bang YJ, Di Bartolomeo M, Mandala M, Ryu MH, et al. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. Lancet. 2018;392(10142):123–133. doi: 10.1016/S0140-6736(18)31257-1. [DOI] [PubMed] [Google Scholar]
- 5.Gopalakrishnan V, Spencer CN, Nezi L, Reuben A, Andrews MC, Karpinets TV, et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science. 2018;359(6371):97–103. doi: 10.1126/science.aan4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillere R, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. 2018;359(6371):91–97. doi: 10.1126/science.aan3706. [DOI] [PubMed] [Google Scholar]
- 7.Cheng WY, Wu CY, Yu J. The role of gut microbiota in cancer treatment: friend or foe? Gut. 2020;69(10):1867–1876. doi: 10.1136/gutjnl-2020-321153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Derosa L, Hellmann MD, Spaziano M, Halpenny D, Fidelle M, Rizvi H, et al. Negative association of antibiotics on clinical activity of immune checkpoint inhibitors in patients with advanced renal cell and non-small-cell lung cancer. Ann Oncol. 2018;29(6):1437–1444. doi: 10.1093/annonc/mdy103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elkrief A, Derosa L, Kroemer G, Zitvogel L, Routy B. The negative impact of antibiotics on outcomes in cancer patients treated with immunotherapy: a new independent prognostic factor? Ann Oncol. 2019;30(10):1572–1579. doi: 10.1093/annonc/mdz206. [DOI] [PubMed] [Google Scholar]
- 10.Chaput N, Lepage P, Coutzac C, Soularue E, Le Roux K, Monot C, et al. Baseline gut microbiota predicts clinical response and colitis in metastatic melanoma patients treated with ipilimumab. Ann Oncol. 2017;28(6):1368–1379. doi: 10.1093/annonc/mdx108. [DOI] [PubMed] [Google Scholar]
- 11.Sivan A, Corrales L, Hubert N, Williams JB, Aquino-Michaels K, Earley ZM, et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science. 2015;350(6264):1084–1089. doi: 10.1126/science.aac4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vetizou M, Pitt JM, Daillere R, Lepage P, Waldschmitt N, Flament C, et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 2015;350(6264):1079–1084. doi: 10.1126/science.aad1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Camilo V, Sugiyama T, Touati E. Pathogenesis of Helicobacter pylori infection. Helicobacter. 2017;22(Suppl):1. doi: 10.1111/hel.12405. [DOI] [PubMed] [Google Scholar]
- 14.Plummer M, Franceschi S, Vignat J, Forman D, de Martel C. Global burden of gastric cancer attributable to Helicobacter pylori. Int J Cancer. 2015;136(2):487–490. doi: 10.1002/ijc.28999. [DOI] [PubMed] [Google Scholar]
- 15.FitzGerald R, Smith SM. An overview of helicobacter pylori Infection. Methods Mol Biol. 2021;2283:1–14. doi: 10.1007/978-1-0716-1302-3_1. [DOI] [PubMed] [Google Scholar]
- 16.Franceschi F, Genta RM, Sepulveda AR. Gastric mucosa: long-term outcome after cure of Helicobacter pylori infection. J Gastroenterol. 2002;37(Suppl 13):17–23. doi: 10.1007/BF02990094. [DOI] [PubMed] [Google Scholar]
- 17.de Korwin JD. Does helicobacter pylori infection play a role in extragastric diseases? Presse Med. 2008;37(3 Pt 2):525–534. doi: 10.1016/j.lpm.2007.07.029. [DOI] [PubMed] [Google Scholar]
- 18.Gravina AG, Zagari RM, De Musis C, Romano L, Loguercio C, Romano M. Helicobacter pylori and extragastric diseases: A review. World J Gastroenterol. 2018;24(29):3204–3221. doi: 10.3748/wjg.v24.i29.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen Y, Blaser MJ. Inverse associations of Helicobacter pylori with asthma and allergy. Arch Intern Med. 2007;167(8):821–827. doi: 10.1001/archinte.167.8.821. [DOI] [PubMed] [Google Scholar]
- 20.Shirzad-Aski H, Besharat S, Kienesberger S, Sohrabi A, Roshandel G, Amiriani T, et al. Association between helicobacter pylori colonization and inflammatory bowel disease: a systematic review and meta-analysis. J Clin Gastroenterol. 2021;55(5):380–392. doi: 10.1097/MCG.0000000000001415. [DOI] [PubMed] [Google Scholar]
- 21.Dellon ES, Peery AF, Shaheen NJ, Morgan DR, Hurrell JM, Lash RH, et al. Inverse association of esophageal eosinophilia with Helicobacter pylori based on analysis of a US pathology database. Gastroenterology. 2011;141(5):1586–1592. doi: 10.1053/j.gastro.2011.06.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elsalem L, Jum'ah AA, Alfaqih MA, Aloudat O. The bacterial microbiota of gastrointestinal cancers: role in cancer pathogenesis and therapeutic perspectives. Clin Exp Gastroenterol. 2020;13:151–185. doi: 10.2147/CEG.S243337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen J, Douglass J, Prasath V, Neace M, Atrchian S, Manjili MH, et al. The microbiome and breast cancer: a review. Breast Cancer Res Treat. 2019;178(3):493–496. doi: 10.1007/s10549-019-05407-5. [DOI] [PubMed] [Google Scholar]
- 24.Wheeler KM, Liss MA. The microbiome and prostate cancer risk. Curr Urol Rep. 2019;20(10):66. doi: 10.1007/s11934-019-0922-4. [DOI] [PubMed] [Google Scholar]
- 25.Yu ZK, Xie RL, You R, Liu YP, Chen XY, Chen MY, et al. The role of the bacterial microbiome in the treatment of cancer. BMC Cancer. 2021;21(1):934. doi: 10.1186/s12885-021-08664-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matson V, Fessler J, Bao R, Chongsuwat T, Zha Y, Alegre ML, et al. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science. 2018;359(6371):104–108. doi: 10.1126/science.aao3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li W, Deng Y, Chu Q, Zhang P. Gut microbiome and cancer immunotherapy. Cancer Lett. 2019;447:41–47. doi: 10.1016/j.canlet.2019.01.015. [DOI] [PubMed] [Google Scholar]
- 28.Park R, Umar S, Kasi A. Immunotherapy in colorectal cancer: potential of fecal transplant and microbiota-augmented clinical trials. Curr Colorectal Cancer Rep. 2020;16(4):81–88. doi: 10.1007/s11888-020-00456-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang C, Wang J, Sun Z, Cao Y, Mu Z, Ji X. Commensal microbiota contributes to predicting the response to immune checkpoint inhibitors in non-small-cell lung cancer patients. Cancer Sci. 2021;112(8):3005–3017. doi: 10.1111/cas.14979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roberti MP, Yonekura S, Duong CPM, Picard M, Ferrere G, Tidjani Alou M, et al. Chemotherapy-induced ileal crypt apoptosis and the ileal microbiome shape immunosurveillance and prognosis of proximal colon cancer. Nat Med. 2020;26(6):919–931. doi: 10.1038/s41591-020-0882-8. [DOI] [PubMed] [Google Scholar]
- 31.Oster P, Vaillant L, Riva E, McMillan B, Begka C, Truntzer C, et al. Helicobacter pylori infection has a detrimental impact on the efficacy of cancer immunotherapies. Gut. 2022;71(3):457–466. doi: 10.1136/gutjnl-2020-323392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bagchi S, Yuan R, Engleman EG. Immune checkpoint inhibitors for the treatment of cancer: clinical impact and mechanisms of response and resistance. Annu Rev Pathol. 2021;16:223–249. doi: 10.1146/annurev-pathol-042020-042741. [DOI] [PubMed] [Google Scholar]
- 33.Arnold IC, Dehzad N, Reuter S, Martin H, Becher B, Taube C, et al. Helicobacter pylori infection prevents allergic asthma in mouse models through the induction of regulatory T cells. J Clin Invest. 2011;121(8):3088–3093. doi: 10.1172/JCI45041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maldonado-Contreras A, Goldfarb KC, Godoy-Vitorino F, Karaoz U, Contreras M, Blaser MJ, et al. Structure of the human gastric bacterial community in relation to Helicobacter pylori status. ISME J. 2011;5(4):574–579. doi: 10.1038/ismej.2010.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heimesaat MM, Fischer A, Plickert R, Wiedemann T, Loddenkemper C, Gobel UB, et al. Helicobacter pylori induced gastric immunopathology is associated with distinct microbiota changes in the large intestines of long-term infected Mongolian gerbils. PLoS ONE. 2014;9(6):e100362. doi: 10.1371/journal.pone.0100362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim ST, Cristescu R, Bass AJ, Kim KM, Odegaard JI, Kim K, et al. Comprehensive molecular characterization of clinical responses to PD-1 inhibition in metastatic gastric cancer. Nat Med. 2018;24(9):1449–1458. doi: 10.1038/s41591-018-0101-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the corresponding author Baicun Hou, upon reasonable request.