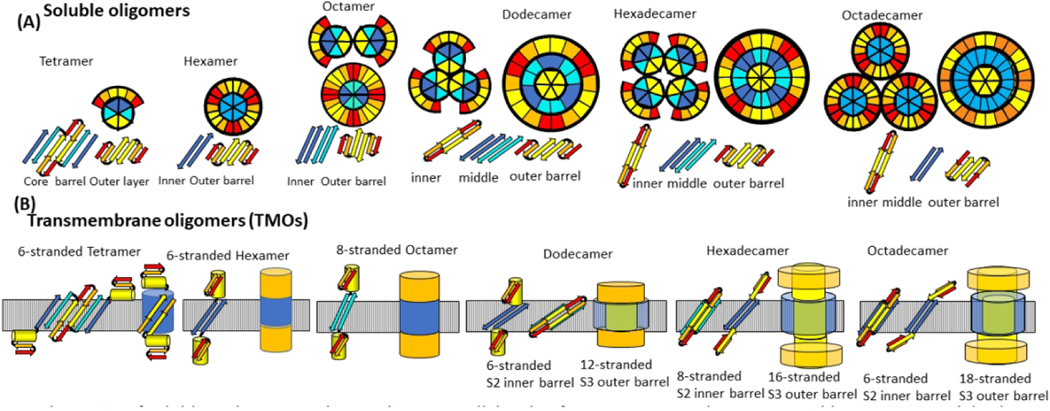
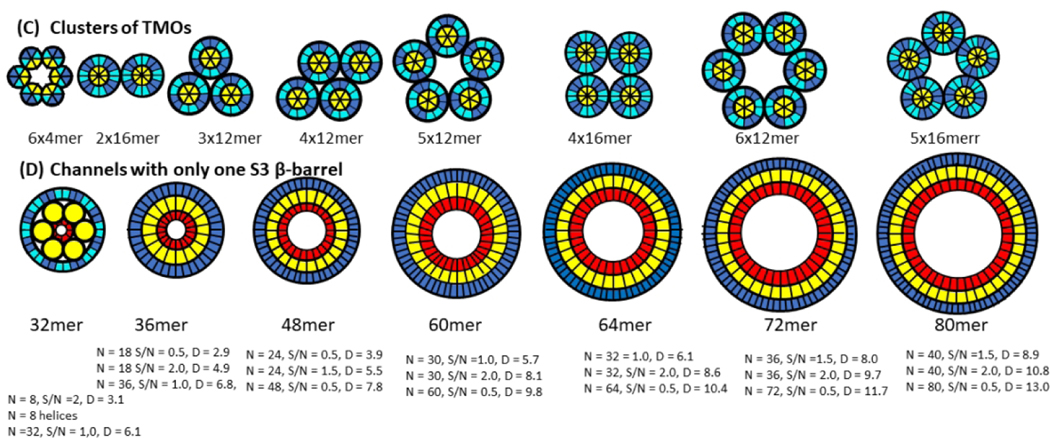
Figure 12.
Hypothesis for how Aβ42 oligomers transition from the aqueous phase to span a membrane, form clusters, and morph into channels with larger pores. (A) Wedge representations and side views of some β-strands colored by segment (red S1a, orange S1b, yellow S2, cyan S3 odd side-chains outward, blue S3 odd side-chains inward). Interactions of tetramers or hexamers are proposed to merge to form larger oligomers. S3 β barrels are sandwiched between an inner S2 barrel and an outer S1-S2 barrel for dodecamers, hexadecamers, and octadecamers in the aqueous phase. (B) Transmembrane octamers (TMOs). Subunit transmembrane topology (small yellow cylinders represent S2 α-helices) and schematic of transmembrane and soluble domains. Transmembrane strands are most tilted for dodecamers and least tilted for octadecamers. Only S3 barrels are in the transmembrane region for hexamers and octamers, S3 transmembrane barrels surround S2 barrels for dodecamers, hexadecamers, and octadecamers. S1 and S2 segments comprise the soluble domains (orange). (C) Wedge schematics of TMO aggregates. (D) Wedge representations of the transmembrane region of channels. An outer S3 barrel surrounds an inner S2 barrel, which may surround S1 barrels. S1 and S2 segments that are not in the transmembrane region form soluble domains (not illustrated). Dimensions and shapes of AFM and freeze-fracture images agree well with dimensions and shapes predicted by these models.