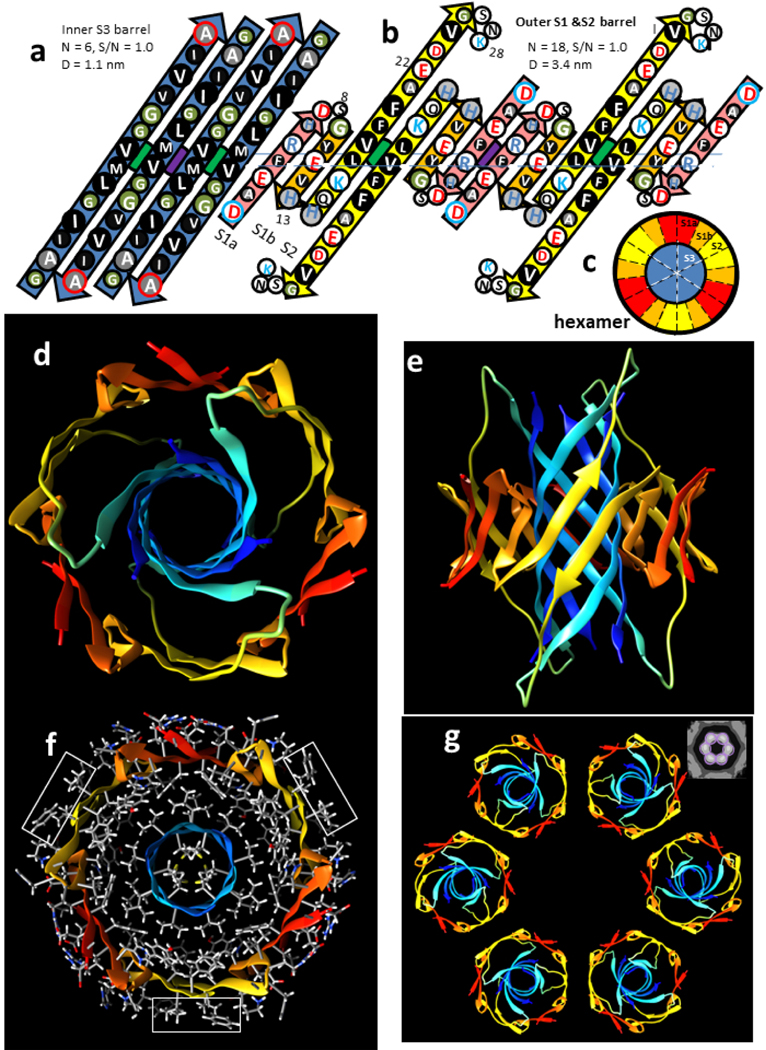
Figure 3.
Hexamer concentric β-barrel model. (a & b) Flattened representation of four subunits viewed from the exterior as if the barrel were split open on the back side and spread flat. The axes of 2-fold perpendicular symmetry are indicated by green and purple circles behind the β-sheets, and the plane containing these axes by the horizontal line. Parameters of the barrels are listed. The arrows represent β-strands colored by segment. The circles represent side chains; those oriented outwardly are larger than those oriented inwardly. They are colored by residue type: white = positively charged (blue letter), negatively charged (red letter), or uncharged polar (black letter), green = ambivalent Gly, light gray = ambivalent His (blue letter) or Thr (black letter), dark gray - white letter = slightly hydrophobic Ala, black - white letter = hydrophobic. The N- and C-termini residues have blue and red outlines to indicate their positive and negative charges. (a) Inner S3 β-barrel. (b) Outer β-barrel; S1a (pink), S1b (orange), and S2 (yellow). (c) Wedge representation cross-section on the plane containing axes of 2-fold symmetry illustrating relative positions of the β-strands. (d-g) Atomic-scale model. The backbone is illustrated as a rainbow-colored ribbon from red (N-termini) to blue (C-termini). (d) View down the radial axis of the β-barrels. (e) Side view along the 2-fold axis between the yellow S2 strands. (f) Radial cross-section through the central portion with side chains colored by element; hydrogens and carbons are white and gray, polar oxygen and nitrogen atoms are red and blue. Rectangles enclose exposed V18 and F20 side chains. (g) Ribbon representations of six hexamers forming a beaded APF; the insert in the upper right corner is an averaged EM image from Fig. 2.