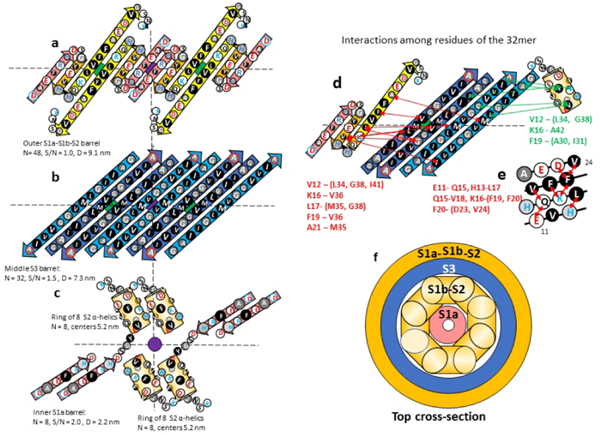
Figure 8.
Model of the 32mer WCsAPF. (a-c) Flattened representation of β-strands for four subunits. The green and purple circles behind the strands indicate the axes of 2-fold perpendicular symmetry and the horizontal dashed line indicates the plane containing those axes. Parameters of the β-barrels are indicated beside or below the schematics. (a) The 3-stranded S1a-S1b-S2 β-sheets that comprise the outer β-barrel. (b) The S3 β-strands of the middle β-barrel. Cout S3 strands are darker blue. (c) The S1b-S2 α-helices and S1b β-strands of two TIM barrel-like structures of Cin subunits. (d) Interactions indicated by NMR studies of Gao et al.39 between residues of S1b-S2 and S3. Red lines and residue list on the left side indicate possible interactions between S1b-S2 residues and outwardly-oriented S3 side chains; green lines and residue list on the right indicate possible interactions between the Cin S1b-S2 α-helix residues and inwardly-oriented S3 side chains. (e) A helical net representation of the S1b-S2 α-helix showing observed interactions among residues that are sequentially separated by three or four positions. (f) Schematic cross-section of the upper portion of the 32-mer model.