The universal guidance of treating patients with type 2 diabetes (T2D) with metformin first has been questioned since positive cardiovascular outcomes trials of antihyperglycemic agents were reported between 2015 and 2021, demonstrating cardiovascular efficacy of multiple glucagon-like peptide-1 receptor agonists and sodium-glucose cotransporter 2 (SGLT2) inhibitors. This underpinned the paradigm shift in the 2019 European Society of Cardiology Guidelines on diabetes and cardiovascular diseases, from a glucose-centric to a risk-driven, evidence-based cardiocentric strategy. Recommendations include glucagon-like peptide-1 receptor agonists or SGLT2 inhibitors with proven cardiovascular benefits as first-line antihyperglycemic therapy in drug-naive patients with T2D and atherosclerotic cardiovascular disease or at high/very high cardiovascular risk.1 The 2022 American Diabetes Association standards of medical care recommend glucagon-like peptide-1 receptor agonists and SGLT2 inhibitors, with or without metformin on the basis of glycemic needs, for those with T2D with or at high risk for atherosclerotic cardiovascular disease, heart failure, or chronic kidney disease.2 Whether background metformin treatment affects the cardiovascular benefits of glucagon-like peptide-1 receptor agonists and SGLT2 inhibitors remains an important question.
Prespecified unadjusted analyses from the VERTIS CV trial (Evaluation of Ertugliflozin Efficacy and Safety Cardiovascular Outcomes, NCT01986881) conducted in patients with T2D and atherosclerotic cardiovascular disease with ertugliflozin suggested no interaction of the presence or absence of baseline metformin on the composite outcome of major adverse cardiovascular events (death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke), cardiovascular death, hospitalization for heart failure, the composite of cardiovascular death/hospitalization for heart failure, or 2 kidney composite end points (doubling of serum creatinine level, kidney replacement therapy, or death from kidney causes; sustained ≥40% decrease in estimated glomerular filtration rate from baseline, kidney replacement therapy, or death from kidney causes).3 However, unadjusted analyses do not take into consideration the differences in baseline clinical characteristics between the subpopulations with and without baseline metformin use, with the corresponding biases and confounding factors that may obscure relevant interactions.
Therefore, to estimate differences in adjusted risk of cardiorenal outcomes between ertugliflozin and placebo across subgroups on the basis of baseline metformin use, we performed post hoc analyses using Cox proportional hazards modeling with propensity adjustment for metformin use by inverse probability of treatment weighting to account for differences in baseline characteristics and risk factor profiles between patients with and without baseline metformin use. The significance level was set to 0.05 for all analyses. VERTIS CV was conducted in accordance with the principles of Good Clinical Practice and was approved by the appropriate institutional review boards and regulatory agencies. Informed consent was obtained from all individuals. On request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions, and exceptions, Pfizer may also provide access to the related individual de-identified participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.
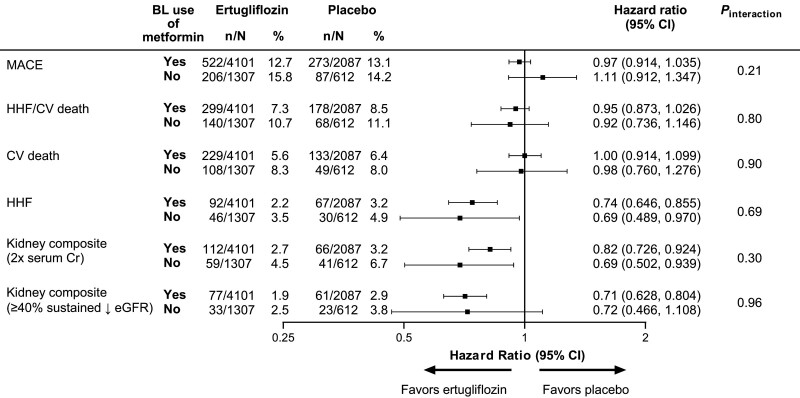
Of 8246 patients in VERTIS CV, 6286 (76%; ertugliflozin: 4164/5499 [75.7%]; placebo: 2122/2747 [77.2%]) used metformin at baseline, alone (n=1149, 18.3%) or with other antihyperglycemic agents. Of those without baseline metformin, 104 (5.4%) were not taking any antihyperglycemic agent. There were notable differences in baseline characteristics in those with, compared with those without, baseline metformin use. These differences included (in those with baseline metformin use): a higher mean estimated glomerular filtration rate (78.1 versus 69.3 mL·min–1·1.73 m–2), fewer patients with estimated glomerular filtration rate <60 mL·min–1·1.73 m–2 (17.9% versus 34.8%), fewer patients on a single antihyperglycemic agent (18.3% versus 76.9%), less insulin use (40.9% versus 67.6%), higher sulfonylurea use (43.8% versus 32.2%), and shorter mean diabetes duration (12.5 versus 14.4 years). As observed in the unadjusted analyses,3 there were no significant propensity-adjusted differences in the effects of ertugliflozin on cardiovascular or kidney outcomes by baseline metformin use (Figure; all pinteraction values >0.2).
Figure.
Cardiovascular and kidney outcomes with ertugliflozin versus placebo by baseline metformin use after propensity adjustment for metformin use. Differences in risk of cardiovascular and kidney outcomes between ertugliflozin and placebo across subgroups by baseline metformin use were estimated from a Cox proportional hazards model by means of propensity adjustment for metformin use by using inverse probability of treatment weighting to account for differences in baseline characteristics and risk factor profiles between patients with and without baseline metformin use. Treatment, baseline metformin use, and the interaction term between treatment and baseline metformin use were included in each model, and the enrollment cohort was included as a stratification factor. Model weights were calculated using propensity score estimates of baseline metformin use and the inverse probability of treatment weighting formula. The variables considered in propensity scoring were age, sex, race, region, body mass index, duration of type 2 diabetes, glycated hemoglobin, total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglycerides, systolic blood pressure, diastolic blood pressure, estimated glomerular filtration rate, and history of: coronary artery disease, cerebrovascular disease, peripheral arterial disease, heart failure, myocardial infarction, coronary artery bypass graft, percutaneous coronary intervention, and stroke. Hazard ratios (95% CIs) are provided for ertugliflozin versus placebo by baseline metformin use. The interaction P value is shown for the 2-level treatment group (all ertugliflozin versus placebo). The kidney composite outcomes were doubling of serum creatinine level, kidney replacement therapy, or death from kidney causes and the exploratory kidney outcome of sustained ≥40% decrease in eGFR from baseline, kidney replacement therapy, or death from kidney causes. BL indicates baseline; Cr, creatinine; CV, cardiovascular; eGFR, estimated glomerular filtration rate; HHF, hospitalization for heart failure; and MACE, major adverse cardiovascular events.
In VERTIS CV, 24% of patients were not on metformin at baseline. This subset of ≈2000 patients not treated with metformin is larger than that of almost every T2D metformin comparator trial before the 2008 regulatory guidance requiring cardiovascular safety assessments for all new medications developed for T2D. In the present analysis, we found no modification of ertugliflozin effect by baseline metformin use on any of the cardiorenal outcomes assessed.
The paradigm shift proposed in the 2019 European Society of Cardiology Guidelines1 has expanded discussion regarding whether metformin should remain first line, because other antihyperglycemic medications have demonstrated cardiorenal benefits in high-risk populations. In the overall VERTIS CV trial, ertugliflozin was noninferior to placebo for major adverse cardiovascular events.4 In addition, there was a 30% relative risk reduction with ertugliflozin in hospitalization for heart failure and a 34% relative risk reduction with ertugliflozin in the prespecified exploratory kidney composite outcome.4,5 In the present analyses with propensity adjustment, the hospitalization for heart failure and kidney outcomes with ertugliflozin were not modified by baseline metformin status. These findings suggest that metformin is unlikely to modulate the benefits of SGLT2 inhibitors on cardiorenal outcomes and that the benefits of SGLT2 inhibitors accrue regardless of metformin use. Given the lack of robust proof of metformin efficacy for cardiovascular outcomes and a less rigorous assessment of cardiovascular safety compared with contemporary antihyperglycemic agents, the elevation of antihyperglycemic agents with proven cardiorenal efficacy and cardiovascular safety above metformin fully represents the application of evidence-based medicine.
Article Information
Registration: URL: https://www.clinicaltrials.gov; Unique identifier: NCT01986881.
Acknowledgments
Editorial support was provided by Dr James of Engage Scientific Solutions (Horsham, UK) and was funded by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, in collaboration with Pfizer Inc., New York, NY.
Sources of Funding
This study was sponsored by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, in collaboration with Pfizer Inc., New York, NY.
Disclosures
Dr Cosentino has received research grants from the Swedish Research Council, Swedish Heart & Lung Foundation, and King Gustav V and Queen Victoria Foundation; and has received consulting fees from Abbott, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Merck Sharp & Dohme, Mundipharma, Novo Nordisk, and Pfizer Inc. Dr Cannon has received research grants from Amgen, Better Therapeutics, Boehringer Ingelheim, Bristol Myers Squibb, Daiichi Sankyo, Janssen, Merck, Novo Nordisk, Pfizer, and fees from Aegerion/Amryt, Alnylam, Amarin, Amgen, Applied Therapeutics, Ascendia, Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly, Janssen, Lexicon, Merck, Pfizer, Rhoshan, and Sanofi; and serves on Data and Safety Monitoring Boards for the Veteran’s Administration, Applied Therapeutics, and Novo Nordisk. Dr Cherney has received consulting fees and speaking honoraria from AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, Janssen, Merck & Co., Inc., Mitsubishi-Tanabe, Novo Nordisk, Prometic, and Sanofi; and has received operating funds from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen, Merck & Co., Inc., Novo Nordisk, and Sanofi. Dr Dagogo-Jack has led clinical trials for AstraZeneca, Boehringer Ingelheim, and Novo Nordisk Inc.; has received consulting fees from AstraZeneca, Bayer, Boehringer Ingelheim, Janssen, Merck & Co., Inc., and Sanofi; and has equity interests in Jana Care Inc. and Aerami Therapeutics. Dr Pratley has received grants (directed to his institution) from Hanmi Pharmaceutical Co., Ltd, Janssen, Metavention, Novo Nordisk, Poxel SA, and Sanofi; has received consulting fees (directed to his institution) from AstraZeneca, Corcept Therapeutics Incorporated, Glytec LLC, Hanmi Pharmaceutical Co., Ltd, Janssen, Merck & Co., Inc., Mundipharma, Novo Nordisk, Pfizer Inc., Sanofi, Scohia Pharma Inc., and Sun Pharmaceutical Industries; and has received support for attending meetings/travel (directed to his institution or to the travel provider) from AstraZeneca, Glytec LLC, Merck & Co., Inc., Mundipharma, Novo Nordisk, and Pfizer Inc. Dr McGuire has received consulting fees from Afimmune, Applied Therapeutics, AstraZeneca, Bayer, Boehringer Ingelheim, Esperion, Lexicon, Lilly USA, Merck Sharp & Dohme, Metavant, Novo Nordisk, Pfizer Inc., and Sanofi US; has received honoraria from Boehringer Ingelheim; has received payment for expert testimony from Kirkland & Ellis on behalf of Boehringer Ingelheim; and has participated on data safety monitoring boards/advisory boards for CSL Behring, AbbVie, Eidos, Otsuka, Arena, and Akebia. Dr Maldonado is an employee of MSD Limited, London, UK, and owns stock in Merck & Co., Inc., Rahway, NJ. Drs Frederich, Mancuso, and Cater are employees and shareholders of Pfizer Inc. Dr Wang was an employee and shareholder of Pfizer Inc. at the time the study was conducted.
Nonstandard Abbreviations and Acronyms
- SGLT2
- sodium-glucose cotransporter 2
- T2D
- type 2 diabetes
- VERTIS CV
- Evaluation of Ertugliflozin Efficacy and Safety Cardiovascular Outcomes
Circulation is available at www.ahajournals.org/journal/circ
This manuscript was sent to Jeffrey Testani, MD, MTR, Guest Editor, for review by expert referees, editorial decision, and final disposition.
This work was presented as an abstract at European Society of Cardiology Congress 2021, The Digital Experience, August 27–30, 2021.
Continuing medical education (CME) credit is available for this article. Go to http://cme.ahajournals.org to take the quiz.
For Sources of Funding and Disclosures, see page 654.
Contributor Information
Christopher P. Cannon, Email: cpcannon@bwh.harvard.edu.
Robert Frederich, Email: robert.frederich@pfizer.com.
David Z.I. Cherney, Email: david.cherney@uhn.on.ca.
Richard E. Pratley, Email: Richard.Pratley.MD@AdventHealth.com.
James P. Mancuso, Email: james.mancuso@pfizer.com.
Mario Maldonado, Email: mario.maldonado@merck.com.
Nilo B. Cater, Email: Nilo.cater@pfizer.com.
Shuai Wang, Email: shuai1107@hotmail.com.
Darren K. McGuire, Email: darren.mcguire@utsouthwestern.edu.
References
- 1.Cosentino F, Grant PJ, Aboyans V, Bailey CJ, Ceriello A, Delgado V, Federici M, Filippatos G, Grobbee DE, Hansen TB, et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2020;41:255–323. doi: 10.1093/eurheartj/ehz486 [DOI] [PubMed] [Google Scholar]
- 2.Draznin B, Aroda VR, Bakris G, Benson G, Brown FM, Freeman R, Green J, Huang E, Isaacs D, Kahan S, et al. 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes–2022. Diabetes Care. 2022;63:S125–S143. doi: 10.2337/dc22-S009 [DOI] [PubMed] [Google Scholar]
- 3.Charbonnel B, Dagogo-Jack S, Cannon CP, Cherney DZI, Cosentino F, McGuire DK, Shih WJ, Liu J, Pong A, Gantz I, et al. 783-P: Cardiorenal outcomes with ertugliflozin by baseline (BL) glucose-lowering agent (GLA): an analysis from VERTIS CV. Diabetes. 2021;70(suppl 1):783-P. doi: 10.2337/db21-783-P [Google Scholar]
- 4.Cannon CP, Pratley R, Dagogo-Jack S, Mancuso J, Huyck S, Masiukiewicz U, Charbonnel B, Frederich R, Gallo S, Cosentino F, et al. Cardiovascular outcomes with ertugliflozin in type 2 diabetes. N Engl J Med. 2020;383:1425–1435. doi: 10.1056/NEJMoa2004967 [DOI] [PubMed] [Google Scholar]
- 5.Cherney DZI, Charbonnel B, Cosentino F, Dagogo-Jack S, McGuire DK, Pratley R, Shih WJ, Frederich R, Maldonado M, Pong A, et al. Effects of ertugliflozin on kidney composite outcomes, renal function and albuminuria in patients with type 2 diabetes mellitus: an analysis from the randomised VERTIS CV trial. Diabetologia. 2021;64:1256–1267. doi: 10.1007/s00125-021-05407-5 [DOI] [PMC free article] [PubMed] [Google Scholar]