FIGURE 6.
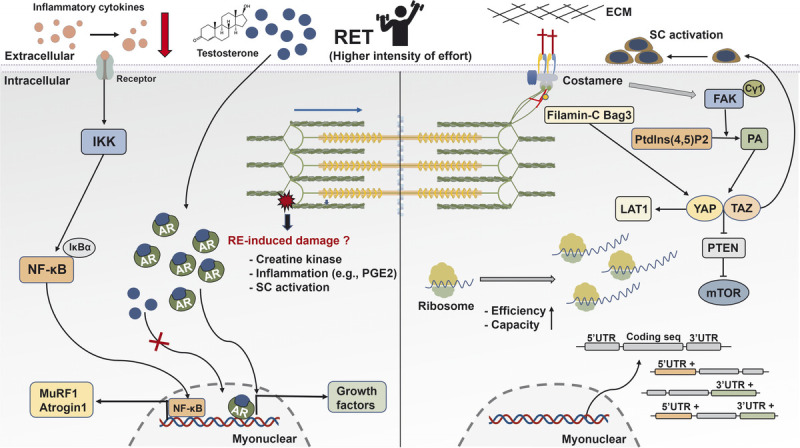
Schematic illustration of the mechanisms underpinning skeletal muscle hypertrophy response to RET. After mechanical stimuli, phospholipase Cγ1 colocalizes around FAK and catalyzes the conversion of Ptdlns(4,5)P2 to PA that activates the HIPPO pathway (YAP and TAZ). YAP and TAZ promote the mTOR signaling pathway via the suppression of PTEN, regulate SC activation, and mediate the expression of L-type amino acid transporter 1 (LAT1). Interaction between Filamin-C and Bag3 increases the activation of the HIPPO pathway. Translational capacity and efficacy via ribosome are essential determinants for muscle hypertrophy. Besides increased gene expression during RE, the UTR of mRNA is regulated and closely correlated with muscle growth. The AR content is associated with RET-induced hypertrophy, whereas RE-induced acute changes in serum testosterone show no association with muscle growth. In human trials, RE-induced increased CK and inflammation factors have no effects on muscle hypertrophy. However, RET reduces resting concentration of inflammation cytokines in aging and clinical illness. AR, androgen receptor; ECM, extracellular matrix; FAK, focal adhesion kinase; IκBα, NF-κB inhibitor alpha; IKK, IκB kinase; LAT1, L-type amino acid transporter 1; mTOR, mechanistic target of rapamycin; MuRF 1, muscle RING-finger protein 1; NF-κB, nuclear factor kappa light-chain enhancer of activated B; PGE2, prostaglandin E2; PTEN, phosphatase and tensin homolog; RE, resistance exercise; RET, resistance exercise training; SC, satellite cells; TAZ, transcriptional coactivator with PDZ-binding motif; UTR, untranslated region; YAP, Yes-associated protein 1.
