Abstract
Background
Despite the rapid adoption of transcatheter aortic valve replacement (TAVR), aortic valve reintervention, particularly surgical TAVR valve explantation (TAVR explant), has not been well described.
Methods
MEDLINE, Embase, and Web of Science were searched through July 2021 to identify observational studies and case series reporting clinical outcomes of TAVR explant. Data on the frequency of TAVR explant, patient demographic characteristics, clinical indications, operative data, and perioperative outcomes were extracted. Study-specific estimates were combined using one-group meta-analysis in a random-effects model.
Results
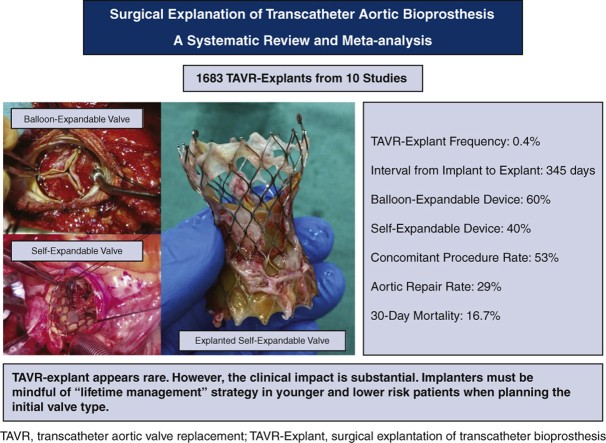
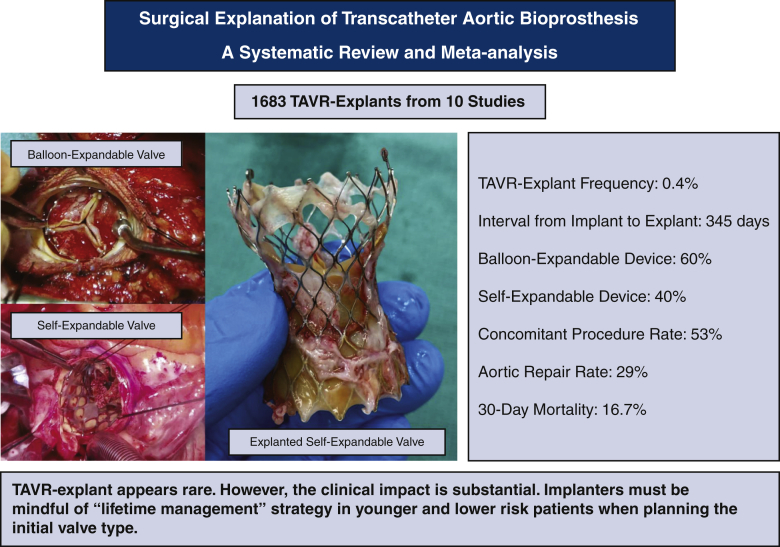
A total of 10 studies were identified that included 1690 patients undergoing a TAVR explant. The frequency of TAVR explant among TAVR recipients was 0.4% (95% confidence interval [CI], 0.2%-0.6%). The mean patient age was 73.7 years (95% CI, 72.9-74.6 years). The mean Society of Thoracic Surgeons predicted risk of mortality was 5.9% (95% CI, 2.9%-8.8%) at the index TAVR and 8.1% (95% CI, 5.4%-10.8%) at TAVR explant. The mean time from implant to explant was 345.0 days (95% CI, 196.7-493.3 days). Among patients with documented device type, 59.8% (95% CI, 43.5%-76.0%) had a balloon-expandable valve and 40.2% (95% CI, 24.0%-56.5%) had a self-expandable valve. Concomitant procedures during TAVR explant were performed in 52.9% of patients (95% CI, 33.8%-72.0%), and the most common concomitant procedure was aortic repair (28.5%; 95% CI, 14.0%-42.9%). The 30-day mortality after TAVR explant was 16.7% (95% CI, 12.2%-21.2%).
Conclusions
TAVR explant in patients with a failing TAVR appears to be rare; however, the clinical impact of TAVR explant is substantial. Implanters must be mindful of the need for a lifetime management strategy in younger and lower-risk patients when choosing the valve type for the initial procedure.
Key Words: transcatheter aortic valve replacement, surgical aortic valve replacement, surgical transcatheter aortic bioprosthesis explantation, structural valve degeneration, reoperative cardiac surgery
Abbreviations and Acronyms: CI, confidence interval; NYHA, New York Heart Association; SAVR, surgical aortic valve replacement; STS-PROM, Society of Thoracic Surgeons predicted risk of mortality; TAVR, transcatheter aortic valve replacement
Graphical abstract
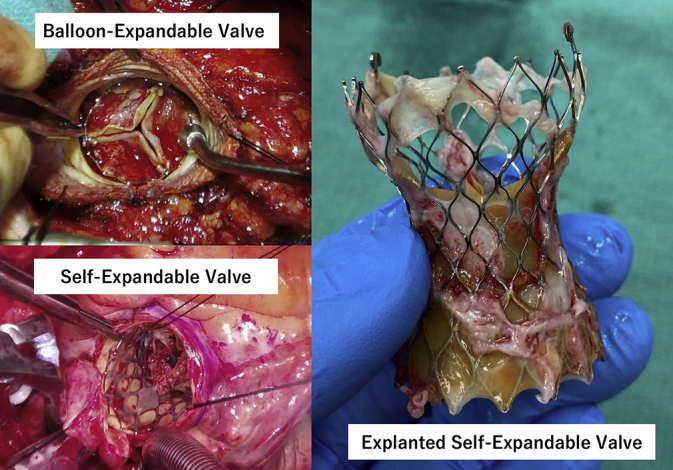
Intraoperative photographs of surgical transcatheter aortic bioprosthesis explantation.
Central Message.
Surgical transcatheter aortic valve replacement (TAVR) valve explantation appears to be rare; however, its mortality and morbidity are substantial. Implanters must be mindful of the need for a lifetime management strategy when choosing candidates for TAVR.
Perspective.
Despite the recent rapid adoption of transcatheter aortic valve replacement (TAVR), surgical TAVR valve explantation (TAVR explant) in patients with a failing TAVR valve appears to be rare. However, the clinical impact of TAVR explant scenario is substantial, with a 30-day mortality approaching 17%. Implanters must be mindful of the need for a lifetime management strategy in younger and lower-risk patients when planning the type of valve for the initial procedure.
Transcatheter aortic valve replacement (TAVR) is an established alternative to surgical aortic valve replacement (SAVR) for patients with severe symptomatic aortic stenosis.1, 2, 3, 4 Its indication has expanded from its original role in a high–surgical risk to a low–surgical risk younger population. At present, the Society of Thoracic Surgeons predicted risk of mortality (STS-PROM) score is not considered a factor in determining candidates for TAVR in patients with suitable anatomy. In addition, the further expansion of TAVR indications now includes bicuspid pathology.5
With the growth in TAVR use in the younger, more healthy patient population, remarkable changes in aortic valve reintervention procedures are expected in the next decade, whereas the frequency, characteristics, and clinical impact of repeat valve intervention remain poorly understood. Only a limited number of studies have described post-TAVR valve reinterventions, either repeat TAVR6,7 or surgical TAVR valve explant (TAVR explant) procedures.8, 9, 10, 11, 12, 13, 14, 15, 16, 17 Of particular concern is the consistent dismal outcomes in patients requiring TAVR explant. In this context, we conducted a systematic review and meta-analysis to better characterize the clinical implications of TAVR explant in patients with a failing TAVR valve using existing evidence and ultimately help refine patient selection for TAVR as the initial valve procedure.
Methods
All observational studies and case series reporting TAVR explant were searched for using a 2-level search strategy. First, PubMed, Embase, and Web of Science were searched through July 31, 2021. Second, relevant studies were identified through a manual search of secondary sources including references of initially identified articles, reviews, and commentaries. All references were downloaded for consolidation, elimination of duplicates, and further analyses. The search terms included “transcatheter aortic valve replacement,” “TAVR,” “explant,” and “reoperation”. Three independent and blinded authors (Y.Y., T.K., and S.F.) reviewed the search results separately to select the studies based on the inclusion and exclusion criteria. Any discrepancies were resolved by discussion and consensus. There were no language restrictions. This study was conducted in accordance with the PRISMA reporting guidelines.18
Studies that met the following criteria were included: the study design was an observational study or a case series, and the study population included adults who underwent TAVR explant. Cases with an intraoperative conversion from TAVR to SAVR were not included. The risk of bias in the individual studies was reviewed using an assessment of the risk of bias in prevalence studies.19
The following information was extracted: authors, year of publication, sample size, frequency of TAVR explant, age, time between TAVR valve implant and explant, STS-PROM, and New York Heart Association (NYHA) class III/IV heart failure at the time of TAVR valve implant and explant, previous cardiac surgery, clinical indications for TAVR explant, type of explanted TAVR valve and implanted surgical valve, concomitantly performed procedures in addition to SAVR, cardiopulmonary bypass and aortic cross-clamp times, 30-day mortality, duration of intensive care unit stay and hospital stay, 30-day readmission, reoperation for bleeding, stroke, renal failure, and new permanent pacemaker implantation. STS-PROM was available only in patients undergoing an STS-PROM calculable procedure, either isolated SAVR or SAVR with coronary artery bypass grafting (CABG). We performed one-group meta-analysis in a random-effects model using the DerSimonian–Laird method for continuous values and the Wald method for discrete values with OpenMetaAnalyst version 12.11.14 (available from http://www.cebm.brown.edu/openmeta/).
Continuous variables are expressed as mean ± SD or median (interquartile range), as appropriate for the data distribution. Categorical variables are expressed as frequency and percentage. Significant heterogeneity was considered present when the I2 index was >50% or the P value for heterogeneity was <.05.
Given the nature of this study, Institutional Research Board approval and patient informed written consent for publication were not required.
Results
Our search identified 1543 articles that were reviewed based on the title and abstract, and of those, 1458 articles were excluded based on title and abstract. In addition, 75 articles were excluded for the following reasons: 35 studies that reported outcomes of SAVR explant, 32 studies that reported outcomes of valve-in-valve replacement, 5 case reports, 2 commentaries, and 1 study with significant duplicated data with other studies.20 Ten articles met the inclusion and exclusion criteria and were included in the meta-analysis8, 9, 10, 11, 12, 13, 14, 15, 16, 17 (Figure E1). Most of the data variables from 2 of these articles9,13 were excluded from the analysis owing to potential cohort duplication with other articles. In addition, for 1 study,17 data for 2012 to 2018 were excluded for the same reason. No cases of intraoperative conversion from TAVR to SAVR were present in the final dataset. Patient characteristics in the included studies are shown in Table E1.
Figure E1.
PRISMA flow chart.
Among the 10 included articles, with a total of 1690 patients, 7 articles were from the United States and 1 article each were from Japan,10 Germany,8 and Italy.16 The frequency of TAVR explants were reported in 4 articles.10,11,14,15 Seven articles presented the age at TAVR explant8,11, 12, 13, 14, 15, 16, 17 and 5 articles included the interval between index TAVR and TAVR explant.8,9,11,12,14 STS-PROM at index TAVR was reported in 3 articles,12, 13, 14 and STS-PROM at TAVR explant also was reported in 3 articles.14,15,17 NYHA class at index TAVR was reported in 2 articles,12,13 and that at TAVR explant was provided in 5 articles.8,13, 14, 15,17 The type of explanted valve was reported in 6 articles.8,12,14, 15, 16, 17 The indication for TAVR explant was reported in 7 articles.8,11,12,14, 15, 16, 17 Data on concomitant procedures at the time of TAVR explant were provided in 7 articles.8,10,11,14, 15, 16, 17 Seven articles reported 30-day mortality.8,11,12,14, 15, 16, 17
The patient characteristics for the meta-analysis are summarized in Table E1. Preoperative data are summarized in Table E2. Indications for TAVR explant are shown in Table E3. Intraoperative data are summarized in Table E4. The postoperative outcomes are shown in Table E5. The results of the pooled analysis are summarized in Table 1. A summary of the risk of bias assessment for the prevalence studies for each retrospective cohort study is provided in Table E6.
Table 1.
Random-effects estimates of patient demographic characteristics, indication, operative data, and perioperative outcomes of the patients with transcatheter valve explant
| Parameter | Pooled estimate (95% CI) | Patients analyzed, n |
|---|---|---|
| Frequency, % | 0.4 (0.2-0.6) | 341,152 |
| Patient characteristics | ||
| Age, y | 73.7 (72.9-74.6) | 1521 |
| STS-PROM at implant | 5.9 (2.9-8.8) | 307 |
| STS-PROM at explant | 8.1 (5.4-10.8) | 992 |
| NYHA III/IV at explant, % | 62.1 (48.9-75.4) | 1009 |
| Previous cardiac surgery, % | 36.8 (10.2-63.4) | 1477 |
| Days from implant to explant, d | 345.0 (196.7-493.3) | 678 |
| Self-expandable valve, % | 40.2 (24.0-56.5) | 907 |
| Balloon-expandable valve, % | 59.8 (43.5-76.0) | 907 |
| Indication | ||
| Endocarditis, % | 37.6 (16.3-58.9) | 1521 |
| SVD | 27.7 (4.8-50.5) | 1501 |
| Paravalvular leak/aortic insufficiency, % | 14.2 (3.3-25.2) | 1501 |
| Failed implantation, % | 12.7 (2.7-22.7) | 1501 |
| Aortic stenosis | 9.1 (0.8-18.9) | 1005 |
| Others | 8.4 (2.9-13.8) | 1501 |
| Operative data | ||
| Isolated explant, % | 47.1 (28.0-66.2) | 1521 |
| Concomitant procedure, % | 52.9 (33.8-72.0) | 1521 |
| Aortic repair, % | 28.5 (14.0-42.9) | 1521 |
| Aortic root repair | 18.8 (7.9-29.7) | 1521 |
| Ascending aortic repair, % | 12.3 (5.4-19.3) | 1521 |
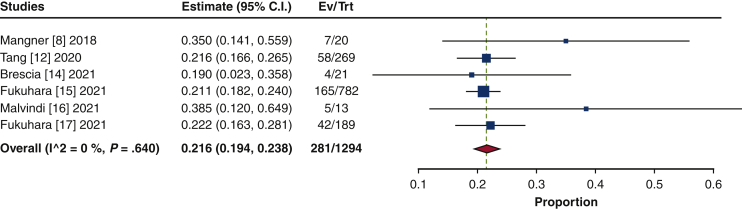
| Mitral valve repair/replacement, % | 21.6 (19.4-23.8) | 1294 |
| CABG, % | 13.8 (10.6-17.1) | 1521 |
| Tricuspid repair/replacement, % | 6.7 (5.3-8.0) | 1274 |
| Bioprosthesis, % | 87.9 (83.7-92.2) | 1213 |
| Mechanical prosthesis, % | 11.8 (8.0-15.7) | 1213 |
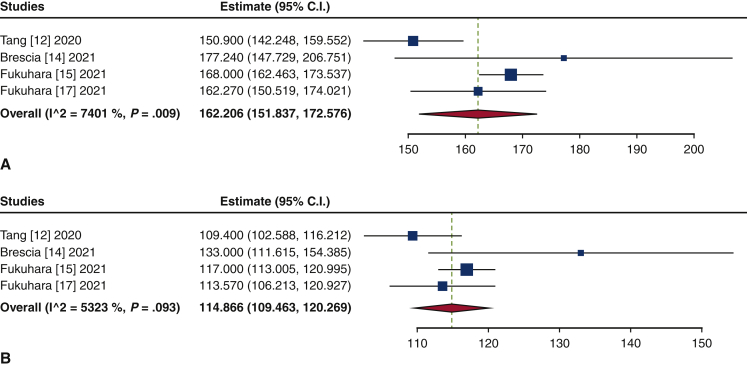
| CPB time, min | 162.2 (151.8-172.6) | 1213 |
| Aortic cross-clamp time, min | 114.9 (109.5-120.3) | 1213 |
| Perioperative outcomes | ||
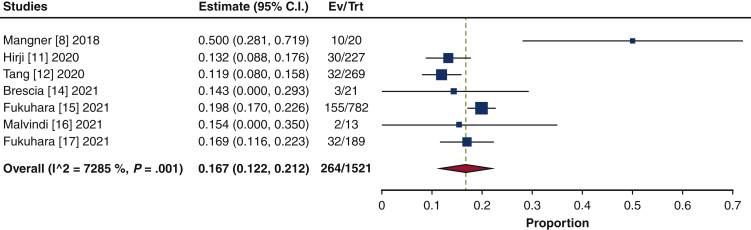
| 30-d mortality, % | 16.7 (12.2-21.2) | 1521 |
| Hospital stay, d | 15.2 (12.1-18.2) | 1488 |
| ICU stay, h | 153.3 (127.8-178.7) | 1488 |
| 30-d readmission, % | 12.7 (10.8-14.6) | 1196 |
| Reoperation for bleeding, % | 8.5 (4.7-12.3) | 1085 |
| Stroke, % | 5.4 (4.2-6.5) | 1501 |
| Renal failure | 16.4 (9.8-23.0) | 1476 |
| New permanent pacemaker insertion, % | 13.1 (10.5-15.8) | 1470 |
CI, Confidence interval; STS-PROM, Society of Thoracic Surgeons predicted risk of mortality; NYHA, New York Heart Association; SVD, structural valvular disease; CABG, coronary artery bypass graft; CPB, cardiopulmonary bypass; ICU, intensive care unit.
Patient Demographic Characteristics
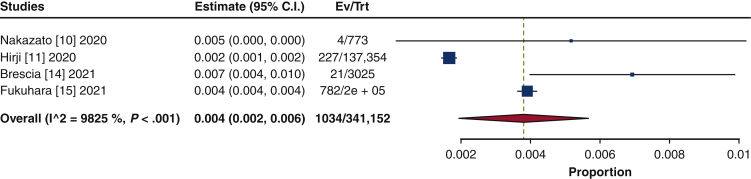
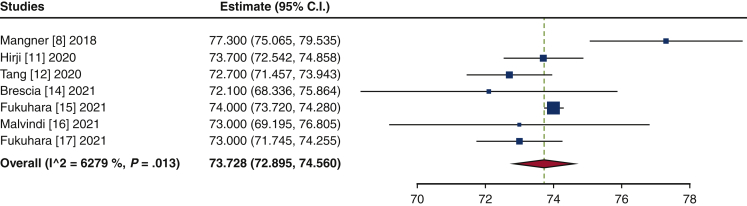
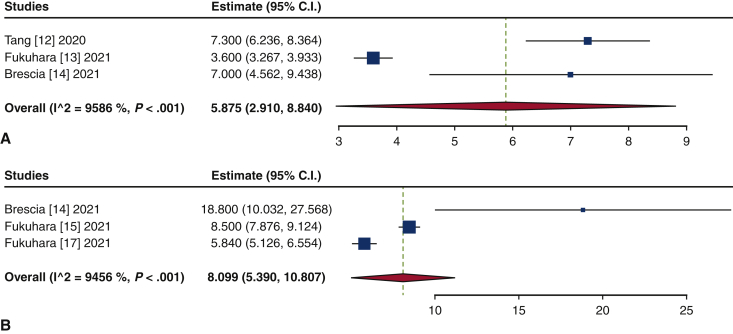
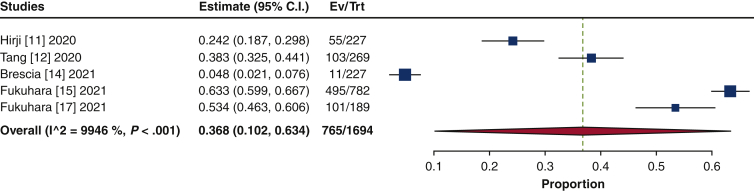
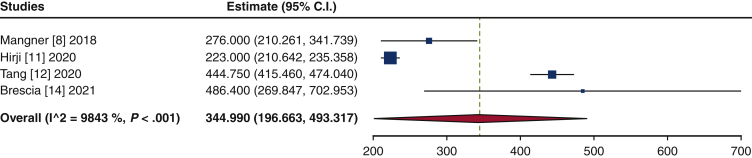
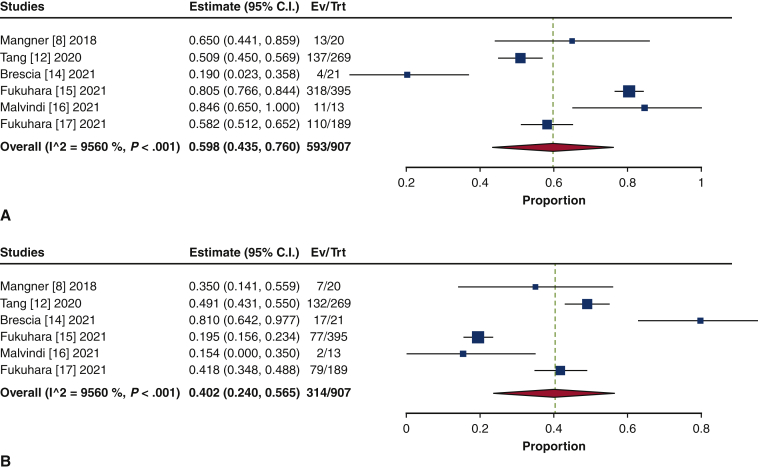
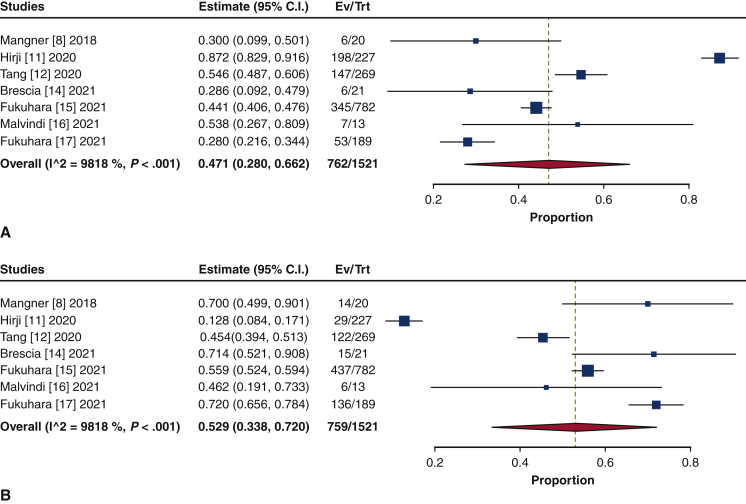
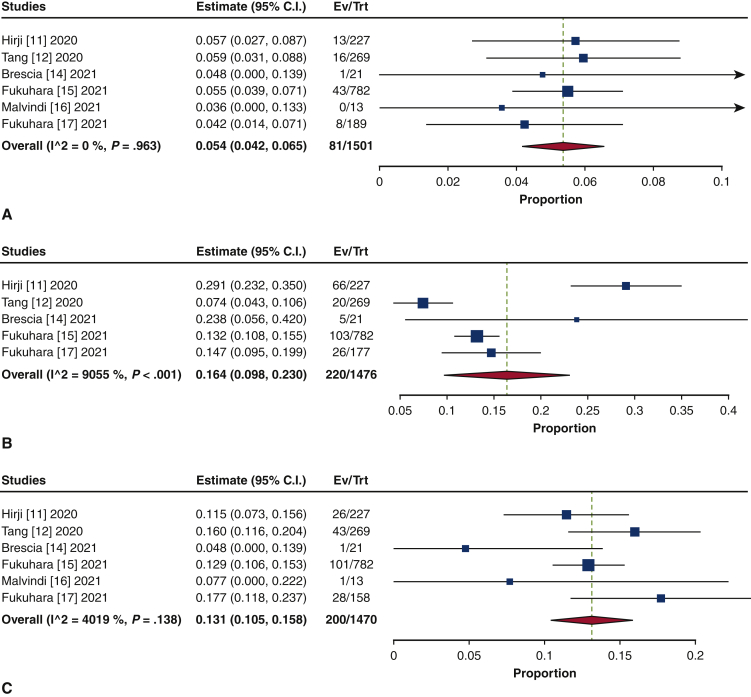
The frequency of TAVR explant procedures among TAVR recipients was 0.4% (95% confidence interval [CI], 0.2%-0.6%; I2 = 98.2%) (Figure 1). The mean patient age at TAVR explant was 73.7 years (95% CI, 72.9-74.6 years; I2 = 62.8%) (Figure E2). The mean STS-PROM was 5.9 (95% CI, 2.9-8.8; I2 = 95.9%) at the time of index TAVR and 8.1 (95% CI, 5.4-10.8; I2 = 94.6%) at TAVR explant (Figure 2). The proportion of patients with NYHA class III/IV heart failure at the time of TAVR explant was 62.1% (95% CI, 48.9%-75.4%; I2 = 89.0%), and 36.8% (95% CI, 10.2%-63.4%; I2 = 99.5%) of patients had a history of previous cardiac surgery (Figure E3). The mean interval from TAVR valve implant to explant was 345.0 days (95% CI, 196.7-493.3 days; I2 = 98.4%) (Figure 3). Among the patients with documented explanted device type, 59.8% (95% CI, 43.5%-76.0%; I2 = 95.6%) had a balloon-expandable valve and 40.2% (95% CI, 24.0%-56.5%; I2 = 95.6%) had a self-expandable valve (Figure E4).
Figure 1.
Forest plots of the included studies showing the pooled estimate of the frequency of transcatheter aortic valve explantations. CI, Confidence interval; EV, number of events; TRT, number of treated.
Figure E2.
Forest plots of the included studies showing the pooled estimate of the age at transcatheter aortic valve explantation. CI, Confidence interval.
Figure 2.
Forest plots of the included studies showing the pooled estimate of the mean Society of Thoracic Surgery predicted risk of mortality (STS-PROM) score at the time of transcatheter aortic bioprosthesis implant (A) and explant (B). CI, Confidence interval.
Figure E3.
Forest plots of the included studies showing the pooled estimate of the proportion of previous cardiac surgery. CI, Confidence interval; EV, number of events; TRT, number of treated.
Figure 3.
Forest plots of the included studies showing the pooled estimate of the mean time interval (days) from transcatheter aortic bioprosthesis implant to explant. CI, Confidence interval.
Figure E4.
Forest plots of the included studies showing the pooled estimate of the proportion of explanted device type: balloon-expandable valves (A) and self-expandable valves (B). CI, Confidence interval; EV, number of events; TRT, number of treated.
Clinical Indications for TAVR Explant
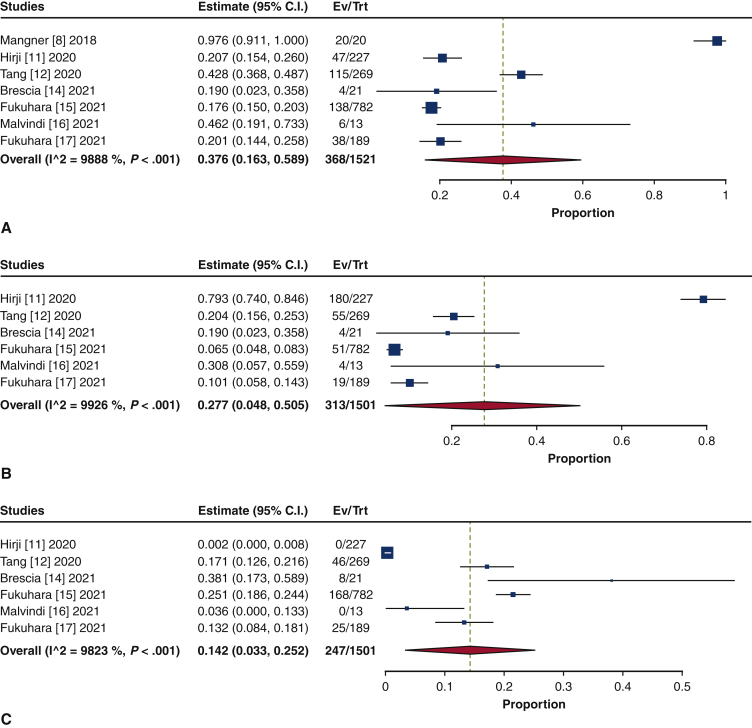
Endocarditis was the most frequent indication for TAVR explant (37.6%; 95% CI, 16.3%-58.9%; I2 = 98.9%) (Figure E5). The second-leading indication was structural valve degeneration (SVD) (27.7%; 95% CI, 4.8%-50.5%; I2 = 99.3%). Other indications included paravalvular leak/aortic insufficiency (14.2%; 95% CI, 3.3%-25.2%; I2 = 98.2%), failed implantation (12.7%; 95% CI, 2.7%-22.7%; I2 = 98.6%), aortic stenosis (9.1%; 95% CI, 0.8%-18.9%; I2 = 94.0%), and others (8.4%; 95% CI, 2.9%-13.8%; I2 = 95.5%).
Figure E5.
Forest plots of the included studies showing the pooled estimates of the proportion of indications for transcatheter valve explantation: endocarditis (A), structured valve degeneration (B), paravalvular leak/aortic insufficiency (C), failed implantation (D), aortic stenosis (E), and others (F). CI, Confidence interval; EV, number of events; TRT, number of treated.
Operative Data
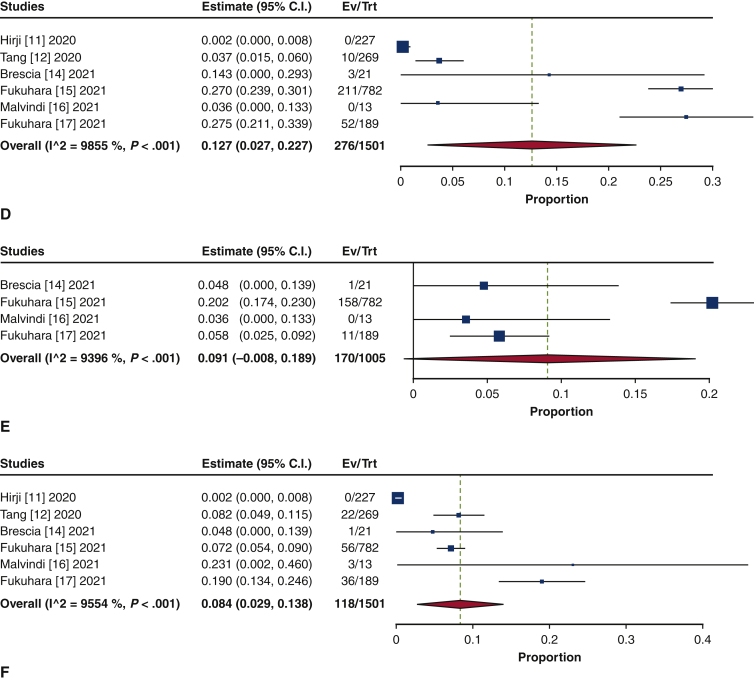
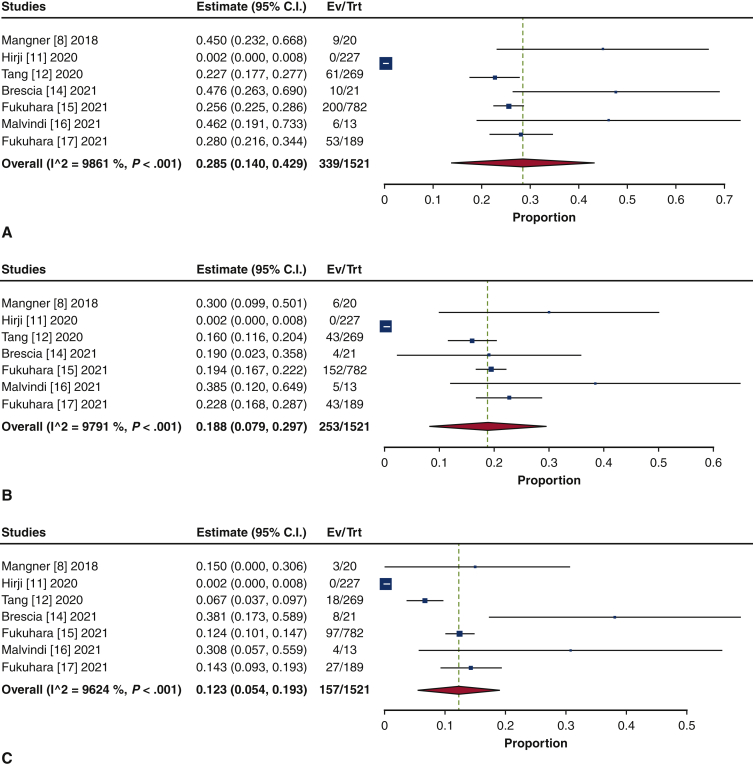
Isolated SAVR was performed in 47.1% of the patients (95% CI, 28.0%-66.2%; I2 = 98.2%), and concomitant procedures were performed in 52.9% (95% CI, 33.8%-72.0%; I2 = 98.2%) (Figure E6). Aortic repair was the most common concomitant procedure (28.5%; 95% CI, 14.0%-42.9%; I2 = 98.6%), comprising aortic root repair (18.8%; 95% CI, 7.9%-29.7%; I2 = 97.9%) and ascending aortic repair (12.3%; 95% CI, 5.4%-19.3%; I2 = 96.2%) (Figure E7). The next most frequently performed procedure was mitral valve repair/replacement (21.6%; 95% CI, 19.4%-23.8%; I2 = 0%) (Figure E8). Other concomitant procedures included CABG (13.8%; 95% CI, 10.6%-17.1%; I2 = 54.6%) and tricuspid repair/replacement (6.7%; 95% CI, 5.3%-8.0%; I2 = 0%) (Figure E9).
Figure E6.
Forest plots of the included studies showing the pooled estimate of the proportions of isolated procedures (A) and concomitant procedures (B). CI, Confidence interval; EV, number of events; TRT, number of treated.
Figure E7.
Forest plots of the included studies showing the pooled estimate of the proportions of concomitant procedures at the time of TAVR explant: aortic repair (A), aortic root repair (B), and ascending aortic repair (C). CI, Confidence interval; EV, number of events; TRT, number of treated.
Figure E8.
Forest plots of the included studies showing the pooled estimate of the proportion of concomitant mitral valve repair/replacement at the time of TAVR explant. CI, Confidence interval; EV, number of events; TRT, number of treated.
Figure E9.
Forest plots of the included studies showing the pooled estimate of the proportion of concomitant procedures at the time of TAVR explant: coronary artery bypass grafting (A) and tricuspid repair/replacement (B). CI, Confidence interval; EV, number of events; TRT, number of treated.
A bioprosthesis was implanted in 87.9% of the patients (95% CI, 83.7%-92.2%; I2 = 68.0%), and a mechanical prosthesis was placed in 11.8% (95% CI, 8.0%-15.7%; I2 = 62.2%) after TAVR explant. The mean cardiopulmonary bypass and aortic cross-clamp times were 162.2 minutes (95% CI, 151.8-172.6 minutes; I2 = 74.0%) and 114.9 minutes (95% CI, 109.5-120.3 minutes; I2 = 53.2%) minutes, respectively (Figure E10).
Figure E10.
Forest plots of the included studies showing the pooled estimates of the mean cardiopulmonary bypass time (minutes) (A) and aortic cross-clamp time (minutes) (B). CI, Confidence interval.
Perioperative Outcomes
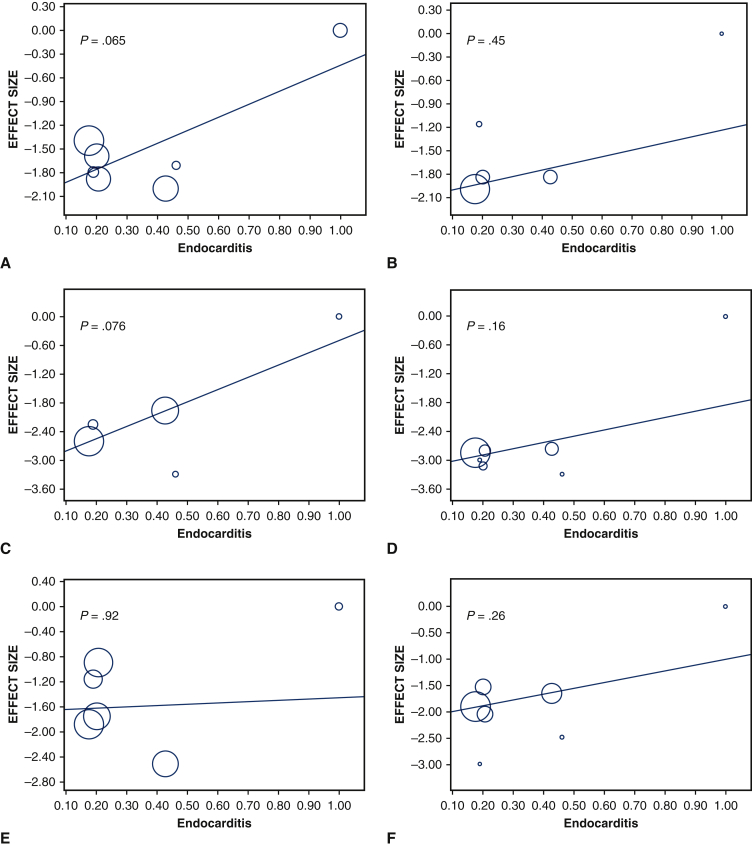
Thirty-day mortality was 16.7% (95% CI, 12.2%-21.2%; I2 = 72.9%) (Figure 4). The mean length of hospital stay was 15.2 days (95% CI, 12.1-18.2 days; I2 = 96.9%), and the mean length of intensive care unit stay was 153.3 hours (95% CI, 127.8-178.7 hours; I2 = 85.9%). The rate of 30-day readmission was 12.7% (95% CI, 10.8%-14.6%; I2 = 0%). Reoperation for bleeding was performed in 8.5% of patients (95% CI, 4.7%-12.3%; I2 = 55.6%). Stroke occurred in 5.4% (95% CI, 4.2%-6.5%; I2 = 0%), renal failure in 16.4% (95% CI, 9.8%-23.0%; I2 = 91%), and new permanent pacemaker insertion in 13.1% (95% CI, 10.5%-15.8%; I2 = 40.2%) (Figure E11). Finally, meta-regression analyses were performed to assess the associations between endocarditis and 30-day mortality, 30-day readmission rate, reoperation for bleeding, stroke, renal failure, and new permanent pacemaker insertion; no significant correlation was identified in any of the outcome measures (Figure E12).
Figure 4.
Forest plots of the included studies showing the pooled estimate of 30-day mortality after surgical transcatheter aortic bioprosthesis explantation. CI, Confidence interval; EV, number of even; TRT, number of treated.
Figure E11.
Forest plots of the included studies showing the pooled estimates of the rates of stroke (A), renal failure (B), and new pacemaker insertion (C). CI, Confidence interval; EV, number of events; TRT, number of treated.
Figure E12.
Meta-regression graph depicting the relationship between propotion of endocarditis and 30-day mortality (A), 30-day readmission (B), reoperation for bleeding (C), stroke (D), renal failure (E), and new permanent pacemaker insertion (F).
Discussion
This study represents the first meta-analysis describing characteristics and outcomes of TAVR explant in patients with a failing TAVR valve. The important findings in the present study were as follows: (1) the overall frequency of TAVR explant without considering competing events was 0.4%; (2) STS-PROM was much higher at the time of TAVR explant than at the time of index TAVR; (3) most TAVR explant cases occurred within 1 year after TAVR; (4) more than one-half of patients underwent a concomitant procedure during TAVR explant; and (5) TAVR explant was associated with significant mortality (Figure 5).
Figure 5.
Meta-analysis of 10 studies including 1690 patients who underwent surgical explantation of transcatheter aortic bioprosthesis. TAVR, Transcatheter aortic valve replacement; TAVR explant, surgical explantation of transcatheter aortic bioprosthesis.
Despite the constant increase in TAVR case volume, the frequency of TAVR explant appears to be low. However, the interpretation of this rarity requires extra caution, for several reasons. First, most patients during these study periods were deemed high/extreme-risk surgical candidates; therefore, it is speculated that not a small number of patients needing valve reintervention without suitable anatomy for repeat TAVR did not undergo a TAVR explant. Second, these TAVR failures were predominantly early failures, occurring within 1-2 years from implantation. Based on our experience with surgical bioprostheses, early bioprosthetic valve failure occurs infrequently within the first 5 years. The underlying pathogenesis of early bioprosthesis failure is distinctly different from that of late failures.21 In addition, paravalvular leak, which is rarely seen in surgical bioprostheses, is one of the most common modes of failure necessitating a TAVR explant. Although the incidence of procedure-related complications such as paravalvular leak are expected to decline with continued refinements in implantation techniques and latest-generation TAVR device, the number of TAVR explant procedures likely will increase when late failures start arising in lower-risk younger patients in the foreseeable future.
Concomitant procedures were frequently performed during many TAVR explant cases, mandating a thorough discussion. This may be explained by several factors, including the presence or exacerbation of uncorrected synchronous cardiac pathologies at the time of the index TAVR procedure, progression of de novo cardiac lesions following the index TAVR, and the need for simultaneous or unplanned procedures due to intraoperative structural injuries resulting from the index TAVR or TAVR explant. Considering the nature of these factors, more thoughtful TAVR candidate selection may be necessary. Synchronous cardiac lesions, such as complex coronary artery and multivalvular heart disease, are known to be common in TAVR recipients. The prevalence of coronary artery disease in TAVR patients ranges from 40% to 75%.22 Although the incidence of acute coronary syndrome necessitating coronary angiography/intervention is seemingly low, percutaneous coronary access and/or intervention in the presence of TAVR can be challenging.23 The prevalence of valvular pathologies, including mitral and tricuspid regurgitation, ranges from 12% to 46% and 11% to 27%, respectively, whereas improvement in regurgitation grade was observed at various rates among studies, and some lesions were reported to worsen after TAVR.24 Concomitant mitral stenosis is documented in up to one-fifth of patients undergoing TAVR and is associated with a 3-fold increased risk of cardiovascular adverse events at 1 year.25 There is clearly a technical learning curve for safe TAVR explant procedures. Tissue trauma and intraoperative complications during TAVR explant become quite infrequent with increasingly more rigorous surgeon experience,26 for which we propose at least 10 to 20 cases with a variety of TAVR device types and clinical indications.
The 30-day mortality was substantially high and was almost 2-fold higher than reported rates of contemporary redo SAVR series.27,28 The worse-than-expected TAVR explant outcomes may be reflective of multiple factors. In addition, although this is a speculative concern, patients with failing TAVR valves might have received an intensive repeat TAVR/nonsurgical intervention (ie, transcatheter closure of paravalvular leak) workup or attempts before the last resort—a TAVR explant. These preceding workup due to the presence of a TAVR valve might have delayed the TAVR explant procedure, resulting in dire clinical outcomes.
This study has several significant limitations. First, only retrospective studies with varying sample sizes were available for the present investigation. Second, there are heterogeneities in definitions of each indication, surgical technique, and complication measurements among studies. In addition, owing to the limited number of TAVR explant studies available, certain clinical characteristics were based solely on just a few study results, and interpretation of these results requires caution. Third, despite the best effort to eliminate the potential cohort duplications from the same database or different data sources, inter-database duplications, such as between Society of Thoracic Surgeons and Center for Medicare & Medicaid Services data, cannot be eliminated completely. Thus, future studies involving alternative non-US data sources are warranted.
Conclusions
Here we have described the clinical impact of TAVR explant in TAVR recipients using available evidence. Although the overall frequency of TAVR explant appears to low, concurrent procedure rates were high at the time of TAVR explant, and the short-term mortality and morbidity were substantial. In this context, it is imperative to focus not only on the periprocedural outcomes following initial TAVR, but also on longer-term considerations for future cardiac reinterventions. These data should be used to more appropriately select candidates for TAVR, especially for younger and lower-risk patients who will likely outlive the longevity of TAVR valves.
Conflict of Interest Statement
Dr Tang has served as a physician proctor for Medtronic and a consultant for Medtronic, Abbott Structural Heart, and W. L. Gore and Associates. Dr Kaneko serves as a speaker for Abbott Structural Heart and Baylis Medical, a consultant for 4C Medical, and has served as a proctor and educator for Edwards Lifesciences and Medtronic. Dr Fukuhara is a consultant for Terumo Aortic. All other authors reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
Appendix E1
Table E1.
Patient characteristics
| Study | Age, y, mean ± SD | Female sex, % | Hypertension, % | Diabetes, % | Dyslipidemia, % | PVD, % | Stroke/TIA, % | COPD, % | CKD, % | Atrial fibrillation, % | AMI, % | CHF, % | Previous PCI, % | Previous CABG, % | Permanent pacemaker, % |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mangner et al, 20188 | 77.3 ± 5.1 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Jawitz et al, 20209 | N/A | 38 | 87 | 36 | N/A | 32 | 10 | N/A | N/A | 33 | N/A | 76 | 32 | 29 | N/A |
| Nakazato et al, 202010 | N/A | 25 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Hirji et al, 202011 | 73.7 ± 8.9 | 35 | 83 | 53 | 72 | 13 | 9 | 35 | 61 | 31 | 7 | 74 | 12 | 24 | N/A |
| Tang et al, 202012 | 72.7 ± 10.4 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Fukuhara et al, 202113 | N/A | 36 | 82 | 24 | 77 | N/A | N/A | 12 | 53 | N/A | N/A | N/A | N/A | N/A | 35 |
| Brescia et al, 202114 | 72 ± 9 | 33 | 89 | 35 | N/A | 20 | 15 | 50 | 13 | N/A | 37 | N/A | N/A | 14 | 28 |
| Fukuhara et al, 202115 | 74 ± 4 | 39 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Malvindi et al, 202116 | 73 ± 7 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 15 | N/A |
| Fukuhara et al, 202117 | 73 ± 8.8 | 38 | 90 | 36 | N/A | N/A | 21 | 18 | N/A | N/A | N/A | N/A | N/A | N/A | 16 |
SD, Standard deviation; PVD, peripheral vascular disease; TIA, transient ischemic attack; COPD, chronic obstructive pulmonary disease; CKD, chronic kidney disease; AMI, acute myocardial infarction; CHF, congestive heart failure; PCI, percutaneous coronary intervention; CABG, coronary artery bypass grafting; N/A, not applicable.
Table E2.
Preoperative data
| Study | Study period | TAVR valve explant, n | TAVR valve implant, n | Days from implant to explant, mean ± SD | STS-PROM at implant, %, mean ± SD | STS-PROM at explant, %, mean ± SD | NHYA III/IV at implant, n | NHYA III/IV at explant, n | Previous cardiac surgery, n | Balloon-expandable valve, n | Self-expandable valve, n |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mangner et al, 20188 | 2008-2017 | 20 | N/A | 276 ± 150 | N/A | N/A | N/A | 16 | N/A | 13 | 7 |
| Jawitz et al, 20209∗ | 2011-2015 | N/A | N/A | 140.3 ± 106.6 | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Nakazato et al, 202010 | 2009-2019 | 4 | 773 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Hirji et al, 202011 | 2012-2017 | 227 | 137,354 | 223 ± 95 | N/A | N/A | N/A | N/A | 55 | N/A | N/A |
| Tang et al, 202012 | 2010-2020 | 269 | N/A | 444.75 ± 245.1 | 7.3 ± 8.9 | N/A | 170 | N/A | 103 | 137 | 132 |
| Fukuhara et al, 202113† | 2011-2019 | N/A | N/A | N/A | 3.6 ± 0.7 | N/A | 14 | 17 | N/A | N/A | N/A |
| Brescia et al, 202114‡ | 2019-2020‡ | 21 | 3025 | 487 ± 690 | 7.3 ± 5.7 | 18.8 ± 20.5 | N/A | 17 | 11 | 4 | 17 |
| Fukuhara et al, 202115 | 2011-2018 | 782 | 200,000 | N/A | N/A | 8.5 ± 8.9 | N/A | 431 | 495 | 318 | 77 |
| Malvindi et al, 202116 | N/A | 13 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 11 | 2 |
| Fukuhara et al, 202117§ | 2019§ | 189 | N/A | N/A | N/A | 5.8 ± 5.0 | N/A | 82 | 101 | 110 | 79 |
TAVR, Transcatheter aortic valve replacement; SD, standard deviation; STS-PROM, Society of Thoracic Surgeons predicted risk of mortality; NYHA, New York Heart Association; N/A, not applicable.
All data were excluded owing to potential duplication with reference 15 except for days from implant to explant variable.
All data were excluded owing to potential duplication with reference 15 except for STS-PROM at implant, NYHA III/IV at implant and explant variables.
Data between 2016 and 2018 were excluded owing to duplication with reference 15.
Table E3.
Indications for TAVR explant
| Study | SVD, n | PVL/aortic insufficiency, n | Aortic stenosis, n | Endocarditis, n | Failed implantation, n |
|---|---|---|---|---|---|
| Mangner et al, 20188 | N/A | N/A | N/A | 20 | N/A |
| Jawitz et al, 20209∗ | N/A | N/A | N/A | N/A | N/A |
| Nakazato et al, 202010 | N/A | N/A | N/A | N/A | N/A |
| Hirji et al, 202011 | 180 | 0 | N/A | 47 | 0 |
| Tang et al, 202012 | 55 | 46 | N/A | 115 | 10 |
| Fukuhara et al, 202113† | N/A | N/A | N/A | N/A | N/A |
| Brescia et al, 202114‡ | 4 | 8 | 1 | 4 | 3 |
| Fukuhara et al, 202115 | 51 | 168 | 158 | 138 | 211 |
| Malvindi et al, 202116 | 4 | 0 | 0 | 6 | 0 |
| Fukuhara et al, 202117§ | 19 | 25 | 11 | 38 | 52 |
TAVR, Transcatheter aortic valve replacement; SVD, structural valve degeneration; PVL, paravalvular leak; N/A, not applicable.
All data were excluded owing to potential duplication with reference 15.
All data were excluded owing to potential duplication with reference 15.
Data between 2016 and 2018 were excluded owing to duplication with reference 15.
Table E4.
Intraoperative data
| Study | Isolated SAVR, n | Concomitant procedures, n |
Mitral repair/replacement, n | CABG, n | Tricuspid repair/replacement, n | Implanted valve type, n |
CPB time, min, mean ± SD | Aortic cross-clamp time, min, mean ± SD | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Aortic repair | Aortic root repair | Ascending aortic repair | Bioprosthesis | Mechanical prosthesis | |||||||
| Mangner et al, 20188 | 6 | 9 | 6 | 3 | 7 | 3 | N/A | N/A | N/A | N/A | N/A |
| Jawitz et al, 20209∗ | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Nakazato et al, 202010 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Hirji et al, 202011 | 198 | 0 | 0 | 0 | N/A | 19 | N/A | N/A | N/A | N/A | N/A |
| Tang et al, 202012 | 147 | 61 | 43 | 18 | 58 | 36 | 24 | 224 | 43 | 150.9 ± 72.4 | 109.4 ± 57.0 |
| Fukuhara et al, 202113† | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Brescia et al, 202114‡ | 6 | 10 | 4 | 8 | 4 | 5 | 2 | 19 | 2 | 177 ± 69 | 133 ± 50 |
| Fukuhara et al, 202115 | 345 | 200 | 152 | 97 | 165 | 122 | 46 | 710 | 72 | 168 ± 79 | 117 ± 57 |
| Malvindi et al, 202116 | 7 | 6 | 5 | 4 | 5 | 2 | 0 | N/A | N/A | N/A | N/A |
| Fukuhara et al, 202117§ | 53 | 53 | 43 | 27 | 42 | 32 | 16 | 124 | 17 | 162.3 ± 83.4 | 113.6 ± 51.6 |
SAVR, Surgical aortic valve replacement; CABG, coronary artery bypass grafting; CPB, cardiopulmonary bypass; SD, standard deviation; N/A, not applicable.
All data were excluded owing to potential duplication with reference 15.
All data were excluded owing to potential duplication with reference 15.
Data between 2016 and 2018 were excluded owing to duplication with reference 15.
Table E5.
Postoperative outcomes
| Study | 30-d mortality, n | Length of hospital stay, d, mean ± SD | Length of ICU stay, h, mean ± SD | 30-d readmission, n | Reoperation for bleeding, n | Stroke, n | Renal failure, n | New permanent pacemaker insertion, n | O/E ratio |
|---|---|---|---|---|---|---|---|---|---|
| Mangner et al, 20188 | 10 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Jawitz et al, 20209∗ | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Nakazato et al, 202010 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Hirji et al, 202011 | 30 | 11.5 ± 2.3 | 126 ± 62 | N/A | N/A | 13 | 66 | 26 | N/A |
| Tang et al, 202012 | 32 | 16.1 ± 13.3 | 147.6 ± 190 | 28 | 33 | 16 | 20 | 43 | 2.51 |
| Fukuhara et al, 202113† | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Brescia et al, 202114‡ | 3 | 15.5 ± 9.2 | 218.2 ± 261.6 | 5 | 2 | 1 | 5 | 1 | 1.54 |
| Fukuhara et al, 202115 | 155 | 16.5 ± 15.6 | 172 ± 224 | 94 | 54 | 43 | 103 | 101 | 1.52 |
| Malvindi et al, 202116 | 2 | N/A | N/A | N/A | 0 | 0 | N/A | 1 | N/A |
| Fukuhara et al, 202117§ | 32 | 16.4 ± 11.9 | 159.2 ± 199.5 | 26 | N/A | 8 | 26 | 28 | 2.74 |
SD, Standard deviation; ICU, intensive care unit; O/E, observed-to-expected; N/A, not applicable.
All data were excluded owing to potential duplication with reference 15.
All data were excluded owing to potential duplication with reference 15.
Data between 2016 and 2018 were excluded owing to duplication with reference 15.
Table E6.
Summary of risk of bias assessment in prevalence studies
| Study | Was the study's target population a close representation of the national population in relation to relevant variables? | Was the sampling frame a true or close representation of the target population? | Was some form of random selection used to select the sample or was a census undertaken? | Was the likelihood of nonresponse bias minimal? | Were data collected directly from the subjects? | Was an acceptable case definition used in the study? | Was the study instrument that measured the parameter of interest shown to have reliability and validity? | Was the same mode of data collection used for all subjects? | Was the length of the shortest prevalence period for the parameter of interest appropriate? | Were the numerators and denominators for the parameter of interest appropriate? | Summary item on the overall risk of study bias |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mangner et al, 20188 | No | No | No | Yes | Yes | Yes | No | Yes | No | Yes | High |
| Jawitz et al, 20209 | No | Yes | No | Yes | Yes | Yes | No | Yes | No | Yes | Moderate |
| Nakazato et al, 202010 | No | Yes | No | Yes | Yes | Yes | No | Yes | No | Yes | Moderate |
| Hirji et al, 202011 | No | Yes | No | Yes | Yes | Yes | No | Yes | No | Yes | Moderate |
| Tang et al, 202012 | No | Yes | No | Yes | Yes | Yes | No | Yes | No | Yes | Moderate |
| Fukuhara et al, 202113 | No | Yes | No | Yes | Yes | Yes | No | Yes | No | Yes | Moderate |
| Brescia et al, 202114 | No | Yes | No | Yes | Yes | Yes | No | Yes | No | Yes | Moderate |
| Fukuhara et al, 202115 | No | Yes | No | Yes | Yes | Yes | No | Yes | No | Yes | Moderate |
| Malvindi et al, 202116 | No | Yes | No | Yes | Yes | Yes | No | Yes | No | Yes | Moderate |
| Fukuhara et al, 202117 | No | Yes | No | Yes | Yes | Yes | No | Yes | No | Yes | Moderate |
Yes = low risk; no = high risk.
Supplementary Data
References
- 1.Leon M.B., Smith C.R., Mack M.J., Makkar R.R., Svensson L.G., Kodali S.K., et al. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2016;374:1609–1620. doi: 10.1056/NEJMoa1514616. [DOI] [PubMed] [Google Scholar]
- 2.Reardon M.J., Van Mieghem N.M., Popma J.J., Kleiman N.S., Søndergaard L., Mumtaz M., et al. Surgical or transcatheter aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2017;376:1321–1331. doi: 10.1056/NEJMoa1700456. [DOI] [PubMed] [Google Scholar]
- 3.Mack M.J., Leon M.B., Thourani V.H., Makkar R., Kodali S.K., Russo M., et al. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med. 2019;380:1695–1705. doi: 10.1056/NEJMoa1814052. [DOI] [PubMed] [Google Scholar]
- 4.Popma J.J., Deeb G.M., Yakubov S.J., Mumtaz M., Gada H., O'Hair D., et al. Transcatheter aortic-valve replacement with a self-expanding valve in low-risk patients. N Engl J Med. 2019;380:1706–1715. doi: 10.1056/NEJMoa1816885. [DOI] [PubMed] [Google Scholar]
- 5.Forrest J.K., Ramlawi B., Deeb G.M., Zahr F., Song H.K., Kleiman N.S., et al. Transcatheter aortic valve replacement in low-risk patients with bicuspid aortic valve stenosis. JAMA Cardiol. 2021;6:50–57. doi: 10.1001/jamacardio.2020.4738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barbanti M., Webb J.G., Tamburino C., Van Mieghem N.M., Makkar R.R., Piazza N., et al. Outcomes of redo transcatheter aortic valve replacement for the treatment of postprocedural and late occurrence of paravalvular regurgitation and transcatheter valve failure. Circ Cardiovasc Interv. 2016;9:e003930. doi: 10.1161/CIRCINTERVENTIONS.116.003930. [DOI] [PubMed] [Google Scholar]
- 7.Landes U., Webb J.G., De Backer O., Sondergaard L., Abdel-Wahab M., Crusius L., et al. Repeat transcatheter aortic valve replacement for transcatheter prosthesis dysfunction. J Am Coll Cardiol. 2020;75:1882–1893. doi: 10.1016/j.jacc.2020.02.051. [DOI] [PubMed] [Google Scholar]
- 8.Mangner N., Woitek F., Haussig S., Schlotter F., Stachel G., Höllriegel R., et al. Incidence, predictors, and outcome of patients developing infective endocarditis following transfemoral transcatheter aortic valve replacement. J Am Coll Cardiol. 2016;67:2907–2908. doi: 10.1016/j.jacc.2016.03.588. [DOI] [PubMed] [Google Scholar]
- 9.Jawitz O.K., Gulack B.C., Grau-Sepulveda M.V., Matsouaka R.A., Mack M.J., Holmes D.R., Jr., et al. Reoperation after transcatheter aortic valve replacement: an analysis of the society of thoracic surgeons database. JACC Cardiovasc Interv. 2020;13:1515–1525. doi: 10.1016/j.jcin.2020.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakazato T., Toda K., Kuratani T., Sawa Y. Redo surgery after transcatheter aortic valve replacement with a balloon-expandable valve. JTCVS Tech. 2020;3:72–74. doi: 10.1016/j.xjtc.2020.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hirji S.A., Percy E.D., McGurk S., Malarczyk A., Harloff M.T., Yazdchi F., et al. Incidence, characteristics, predictors, and outcomes of surgical explantation after transcatheter aortic valve replacement. J Am Coll Cardiol. 2020;76:1848–1859. doi: 10.1016/j.jacc.2020.08.048. [DOI] [PubMed] [Google Scholar]
- 12.Tang G., Sengupta A., Zaid S., Vitanova K., Lange R., Munsterer A., et al. TCT CONNECT-4 Surgical EXPLANTation after transcatheter aortic valve replacement failure: midterm outcomes from the EXPLANT-TAVR International Registry. J Am Coll Cardiol. 2020;76(17 Suppl S):B2–B3. [Google Scholar]
- 13.Fukuhara S., Brescia A.A., Shiomi S., Rosati C.M., Yang B., Kim K.M., et al. Surgical explantation of transcatheter aortic bioprostheses: results and clinical implications. J Thorac Cardiovasc Surg. 2021;162:539–547.e1. doi: 10.1016/j.jtcvs.2019.11.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brescia A.A., Deeb G.M., Sang S.L.W., Tanaka D., Grossman P.M., Sukul D., et al. Surgical explantation of transcatheter aortic valve bioprostheses: a statewide experience. Circ Cardiovasc Interv. 2021;14:e009927. doi: 10.1161/CIRCINTERVENTIONS.120.009927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fukuhara S., Brescia A.A., Deeb G.M. Surgical explantation of transcatheter aortic bioprostheses: an analysis from the Society of Thoracic Surgeons database. Circulation. 2020;142:2285–2287. doi: 10.1161/CIRCULATIONAHA.120.050499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malvindi P.G., Lorusso R., Jiritano F., Santarpino G., Pilato M., Cammardella A.G., et al. Late surgical treatment for transcatheter aortic valve prosthesis dysfunction. Ann Thorac Surg. 2021;111:e271–e273. doi: 10.1016/j.athoracsur.2020.06.138. [DOI] [PubMed] [Google Scholar]
- 17.Fukuhara S., Nguyen C.T.N., Yang B., Patel H.J., Ailawadi G., Kim K.M., et al. Surgical explantation of transcatheter aortic bioprostheses: balloon versus self-expandable devices. Ann Thorac Surg. February 3, 2021 doi: 10.1016/j.athoracsur.2021.01.041. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 18.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6 e1000097. [PMC free article] [PubMed] [Google Scholar]
- 19.Kim S.Y., Park J.E., Lee Y.J., Seo H.J., Sheen S.S., Hahn S., et al. Testing a tool for assessing the risk of bias for nonrandomized studies showed moderate reliability and promising validity. J Clin Epidemiol. 2013;66:408–414. doi: 10.1016/j.jclinepi.2012.09.016. [DOI] [PubMed] [Google Scholar]
- 20.Ando T., Adegbala O., Aggarwal A., Afonso L., Takagi H., Grines C.L., et al. Redo aortic valve intervention after transcatheter aortic valve replacement: analysis of the nationwide readmission database. Int J Cardiol. 2021;325:115–120. doi: 10.1016/j.ijcard.2020.10.038. [DOI] [PubMed] [Google Scholar]
- 21.Cremer P.C., Rodriguez L.L., Griffin B.P., Tan C.D., Rodriguez E.R., Johnston D.R., et al. Early bioprosthetic valve failure: mechanistic insights via correlation between echocardiographic and operative findings. J Am Soc Echocardiogr. 2015;28:1131–1148. doi: 10.1016/j.echo.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 22.Faroux L., Guimaraes L., Wintzer-Wehekind J., Junquera L., Ferreira-Neto A.N., Del Val D., et al. Coronary artery disease and transcatheter aortic valve replacement: JACC state-of-the-art review. J Am Coll Cardiol. 2019;74:362–372. doi: 10.1016/j.jacc.2019.06.012. [DOI] [PubMed] [Google Scholar]
- 23.Yudi M.B., Sharma S.K., Tang G.H.L., Kini A. Coronary angiography and percutaneous coronary intervention after transcatheter aortic valve replacement. J Am Coll Cardiol. 2018;71:1360–1378. doi: 10.1016/j.jacc.2018.01.057. [DOI] [PubMed] [Google Scholar]
- 24.Khan F., Okuno T., Malebranche D., Lanz J., Praz F., Stortecky S., et al. Transcatheter aortic valve replacement in patients with multivalvular heart disease. JACC Cardiovasc Interv. 2020;13:1503–1514. doi: 10.1016/j.jcin.2020.03.052. [DOI] [PubMed] [Google Scholar]
- 25.Asami M., Windecker S., Praz F., Lanz J., Hunziker L., Rothenbühler M., et al. Transcatheter aortic valve replacement in patients with concomitant mitral stenosis. Eur Heart J. 2019;40:1342–1351. doi: 10.1093/eurheartj/ehy834. [DOI] [PubMed] [Google Scholar]
- 26.Fukuhara S. Safe late explantation of transcatheter aortic bioprosthesis. Ann Thorac Surg. 2020;110:e555–e558. doi: 10.1016/j.athoracsur.2020.04.089. [DOI] [PubMed] [Google Scholar]
- 27.Kaneko T., Vassileva C.M., Englum B., Kim S., Yammine M., Brennan M., et al. Contemporary outcomes of repeat aortic valve replacement: a benchmark for transcatheter valve-in-valve procedures. Ann Thorac Surg. 2015;100:1298–1304. doi: 10.1016/j.athoracsur.2015.04.062. [DOI] [PubMed] [Google Scholar]
- 28.Erlebach M., Wottke M., Deutsch M.A., Krane M., Piazza N., Lange R., et al. Redo aortic valve surgery versus transcatheter valve-in-valve implantation for failing surgical bioprosthetic valves: consecutive patients in a single-center setting. J Thorac Dis. 2015;7:1494–1500. doi: 10.3978/j.issn.2072-1439.2015.09.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.