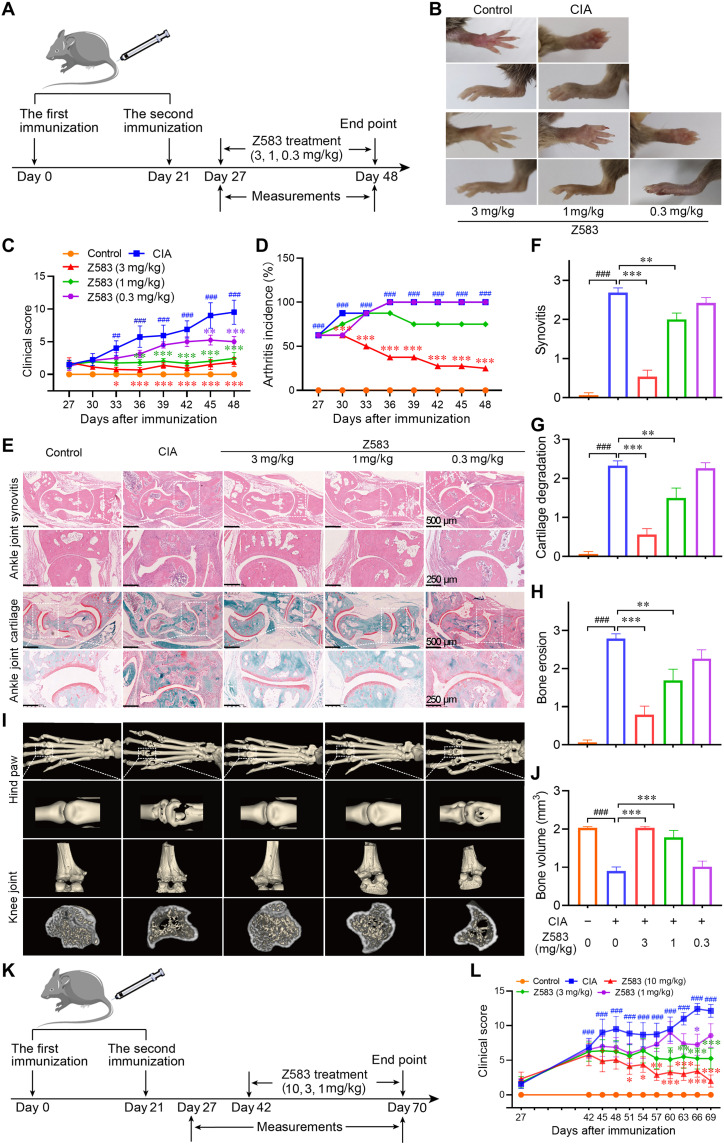
Fig. 6. Selective JAK3 inhibitor Z583 treatment alleviated CIA in mice.
(A) Experimental scheme of the prophylactic effect of Z583 for the analysis of CIA; DBA/1 mice were immunized on days 0 and 21 and were treated with different doses of Z583 (3, 1, and 0.3 mg/kg) from day 27 for 3 weeks. (B) Representative hind paws from each treatment group. Clinical score (C) and CIA incidence (D) were assessed every 3 days in control, CIA mice, and Z583-treated CIA mice. (E) Ankle joint sections were stained by hematoxylin and eosin (H&E) or saffron O/Fast green staining. Quantification of synovitis (F), cartilage degradation (G), and bone erosion (H) in ankle joint was assessed using clinical scores on day 48. (I) Representative micro-CT images of hind paws and knees. (J) Bone volume of the right hind paw second to fourth metatarsophalangeal joint was assessed using Mimics software. (K) Experimental scheme of the therapeutic effect of Z583 for the analysis of CIA. DBA/1 mice were immunized on days 0 and 21 and were treated with different doses of Z583 (10, 3, and 1 mg/kg) from day 43 for 4 weeks. (L) Clinical score was observed every 3 days in control, CIA mice, and Z583-treated CIA mice. Data are presented as means ± SEM, n = 8. ##P < 0.01 and ###P < 0.001 versus control group; *P < 0.05, **P < 0.01, and ***P < 0.001 versus CIA group.