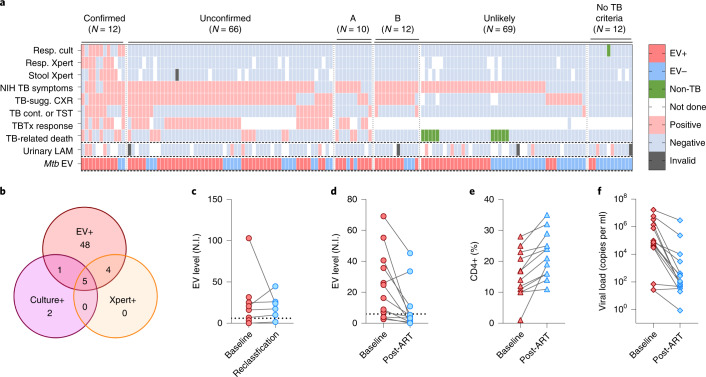
Fig. 3. Mtb EV NEI diagnostic performance in children living with HIV at high risk of TB.
a, NEI signal in children with confirmed, unconfirmed and unlikely TB as determined by positive respiratory culture/Xpert or stool Xpert results, TB-related symptoms meeting NIH criteria for the duration, chest X-ray (CXR) findings, close TB contact or positive TST, positive TBTx response, and/or TB-related death. Urine LAM results and serum Mtb EV results were not used for classification. Subgroup A: children reclassified from unlikely to unconfirmed TB on the basis of TBTx (TB treatment) response or TB-related death as determined by an expert review panel (see Supplementary Table 7 for criteria). Subgroup B: children reclassified from unconfirmed to unlikely TB on the basis of symptom improvement following ART initiation without TBTx initiation (alternate data for anti-TBTx response). b, TB cases classified by NEI, Mtb culture and/or Xpert test results. c, Positive baseline NEI signal predicts subsequent TB reclassification in children with unlikely TB assignments at enrolment (Subgroup A in a) who were reclassified to unconfirmed TB by investigators blinded to EV results. d–f, NEI signal decreases (d) among children diagnosed as unconfirmed TB cases at baseline but reclassified as unlikely TB cases due to symptom improvement without TBTx following ART initiation alone (Subgroup B in a), which corresponded with CD4 cell % increases (e) and HIV viral load reductions (f) following ART initiation.