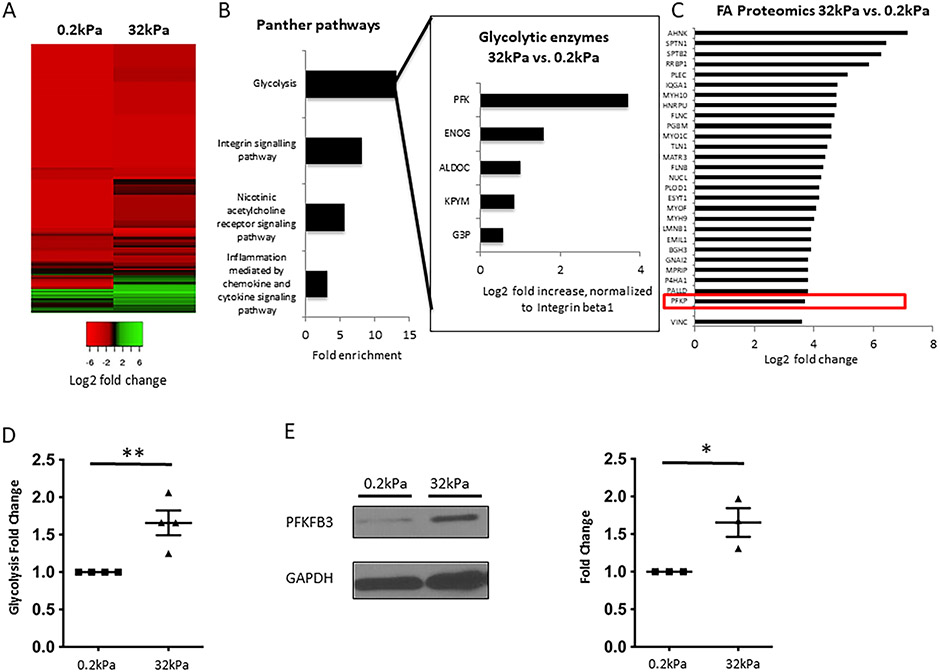
Fig. 1. Increased stiffness results in formation of focal adhesions and recruitment of glycolytic enzymes, particularly Phoshofructokinase 1, to focal adhesions.
A. Heatmap for isolated FA proteins from human LSEC plated on hard (32kPa) vs. soft gel (0.2kPa), log2 fold change compared to integrin beta 1 (relative abundance), based on total spectrum counts obtained from mass spectrometry. Green color indicates relative enrichment of FA proteins compared to Integrin beta 1 (main protein within focal adhesions), red color indicates that the FA protein is less abundant than Integrin beta 1. Heatmap reveals considerable differences in the composition of FA on hard vs. soft gels. B. Gene ontology analysis of FA proteins upregulated ≥1.5 fold on hard vs. soft gels reveals glycolysis as the most enriched pathway, which is more enriched than the Integrin signaling pathway. Insert shows glycolytic enzymes contributing to the enrichment of glycolysis and their log2 fold increase on hard vs. soft gels. All values are normalized to Integrin beta 1. PFK is identified as the most upregulated glycolytic enzyme in FA on hard vs. soft gels. C. Mass spectrometry revealed upregulation of typical FA proteins in FA isolates from human LSEC plated on hard vs. soft gels, with upregulation of PFK being comparable to these typical FA proteins. Values represent log2 fold change of total spectrum counts on hard vs. soft gels, normalized to Integrin beta 1. D. Glycolysis was significantly increased in human LSEC plated on hard gels compared to soft gels using radiolabeled glucose experiments (n=4, **p<0.01). E. Protein levels of PFKFB3, a key activator of glycolysis, are significantly upregulated in human LSEC seeded on hard vs. soft gels (western blots on the left, quantification on the right, n=3, *p<0.05), GAPDH was used as a housekeeping cytosolic gene without changes in the whole cell lysate.