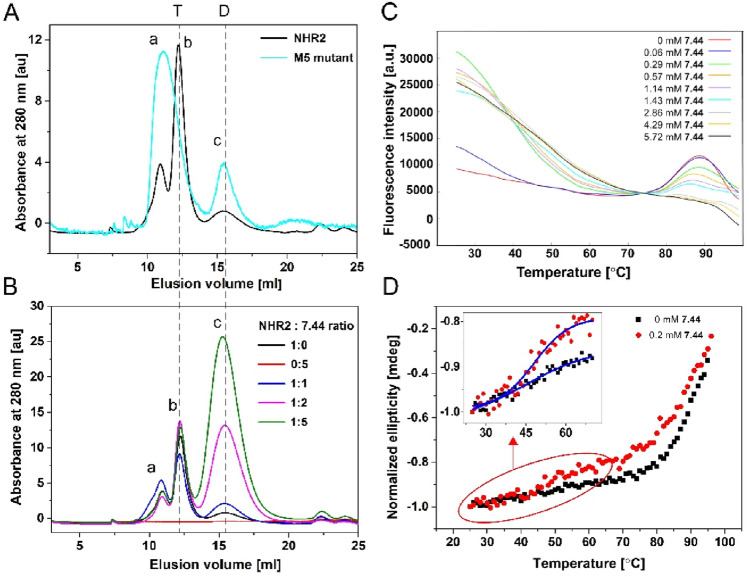
Figure 2.
Stability of the NHR2 tetramer with and without 7.44 studied by size exclusion chromatography, DSF, and CD spectroscopy. (A) Elution profiles of purified proteins of the NHR2 domain (black) and M5 variant (cyan) from size exclusion chromatography (SEC) obtained at 280 nm absorbance: higher-order oligomers (a), tetramer (b), and dimer (c). The integration of peaks (b) and (c) of the NHR2 domain yields 92% of the tetramer and 8% of the dimer. (B) Elution profiles of 30 µM of apo NHR2 (black), 150 µM of 7.44, and NHR2:7.44 with molar ratios of 1:1 (blue), 1:2 (magenta), and 1:5 (green) obtained from SEC at 280 nm. Samples were incubated at 37 °C for 2 h and then incubated overnight at room temperature prior to the experiment. (C) Fluorescence intensity in the presence of 7.44 at different concentrations; for better visualization, the curves were shifted along the y-axis to match them at the point between the two melting points of dimer and tetramer. (D) Thermal denaturation of apo NHR2 (black) and NHR2 in the presence of 7.44 (red) monitored by CD spectroscopy at 222 nm wavelength. The CD data was normalized with respect to the highest ellipticity value for better comparison.